4047
Feasibility of MR guided High Intensity Focused Ultrasound (MRgHIFU) for treating recurrent gynecological tumours: a pilot study1CRUK Cancer Imaging Centre, Institute of Cancer Research, London, United Kingdom, 2CRUK Cancer Imaging Centre, Royal Marsden Hospital, London, United Kingdom, 3Therapeutic Ultrasound, Institute of Cancer Research, London, United Kingdom, 4Gynae-Oncology, Royal Marsden Hospital, London, United Kingdom
Synopsis
MR guided high intensity focused ultrasound (MRgHIFU) treatment plans were generated for 16 patients with recurrent gynecological tumors. Treatment volumes (i) ignoring risk to non-target regions (TVunconstrained), (ii) considering risk, assuming that patients were optimally prepared (TVoptimal), and (iii) assuming no preparation was possible (TVno-prep), were compared with planning target volume (PTV). 9/16 patients (56%) with tumor volumes ≤53 ml were considered feasible to treat safely if optimally prepared (TVoptimal≥50% PTV). Main limiting factors were transducer focal range, bone in the beam path, and risk to bowel. However, even without preparation, 4/16 patients (25%) remained feasible to treat (TVno-prep ≥50% PTV).
Background
Recurrent gynecological malignancies carry a poor prognosis, and on-going symptoms of pain and bleeding substantially negatively impact patients’ quality of life1,2. Treatment options can be limited, particularly when there has been prior radiation therapy3,4. As MR guided high intensity focused ultrasound (MRgHIFU) can be delivered without risking additional radiation toxicity, it offers an alternative treatment option in these patients, and has been used for many years to treat benign gynecological disease5. However, evidence for its use for treating gynecological cancer comprises a single case study6, and the feasibility of delivering MRgHIFU based on anatomical location and volume of recurrence has not been investigated.Purpose
To investigate the feasibility of using MRgHIFU to treat recurrent gynecological tumors, using a Philips 3T Achieva MR/Sonalleve HIFU clinical system (NCT02714621)7.Methods
Patients: 16 patients with recurrent gynecological malignancy (9 vagina or vault, 2 uterus, 2 adnexa, 2 pelvic sidewall, 1 inguinal region) provided written, informed consent to participate in this feasibility study, without progressing to treatment.
Imaging: Patients were positioned on the Sonalleve table in a potential treatment position, with recurrent disease close to the centre of the homed transducer. Slice-matched T2W, T1W, and diffusion-weighted (DW) sequences were acquired using HIFU window and pelvic coils in combination, and exported to the Sonalleve treatment planning system.
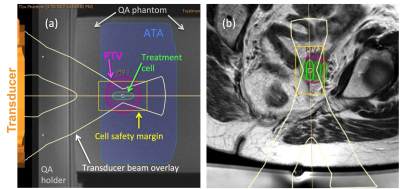
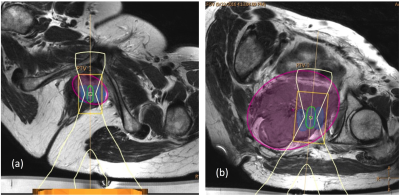
Defining the treatment region: The treatment region was defined by manually outlining an ellipsoid planning target volume (PTV). The PTV encompassed gross tumor volume (GTV) seen on MR imaging, but was not conformal to it, if GTV was irregularly shaped.
Measuring treatment volume: Appropriately sized treatment cells8 (4,8,12,14, or 16 mm diameter) were manually prescribed to provide maximum coverage of the PTV, ignoring acoustically opaque regions, or risk to heat-sensitive non-target regions. Total volume of these cells provided the unconstrained treatment volume (TVunconstrained) (Figure 1). Cells were then safety-checked to determine whether: (i) bone formed an acoustic obstacle in the beam path, (ii) major nerves were within the cell safety margin9, and (iii) there was risk of exposure to bladder or bowel. This assessment was made under 2 opposing assumptions: (a) bladder and/or bowel preparation (e.g. bladder filling, aspiration of rectal air, or rectal filling with acoustic coupling medium10, 11), could be used to avoid damage if these organs were located in the near or far field, but not if they were within the cell safety margin (TVoptimal), or (b) no interventional preparation was possible (TVno-prep).
Feasibility Assessment: TVs were expressed as a percentage of PTV. A patient was considered feasible to treat if TVoptimal was ≥50% of PTV, as data from fibroid treatments have indicated response effects beyond the actual treatment volume12. Where TVunconstrained was <100% PTV, the difference value quantified the proportion of PTV beyond the focal range of the transducer. The volume of safety-checked cells restricted by bone, nerves, bladder, or bowel were expressed as a percentage of PTV for both TVoptimal and TVno-prep.
Results
PTV was always larger than GTV: mean±SD percentage difference = 22.9±14.6% (range: 4.3-55.3%).
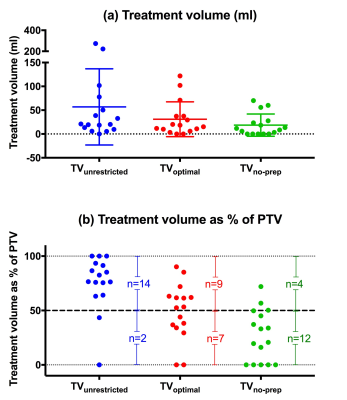
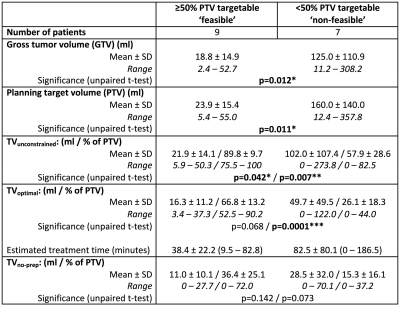
TVunconstrained, TVoptimal and TVno-prep for all 16 patients are shown in Figure 2 as (a) volumes, and (b) percentage of PTV. This indicates that 9/16 recruited patients (56%) with tumor volumes ≥53 ml had >50% of the tumor accessible to treatment if optimally prepared (Figure 3). Differences in treatment plan metrics between ‘feasible’ (TVoptimal ≥50% PTV) and ‘non-feasible’ (TVoptimal <50% PTV) patients are given in Table 1.
TVunconstrained was <100% in 13/16 patients; the mean±SD proportion of the PTV beyond the focal range of the transducer was 21±27 %. In optimally prepared patients, the proportion of PTV [mean±SD, (range)] that could not be targeted due to (i) bone was 25±20% (0-50%), (ii) major nerves was 5±10% (0-34%), and (iii) bladder or bowel was 1±4% (0-14%). Without interventional preparation, 22±23 % (0-75%) of PTVs was considered non-targetable due to risk to bowel, and 2±5% (0-15%) due to risk to bladder.
Discussion and Conclusion
MRgHIFU treatment was considered feasible in >50% of recruited patients if they were optimally prepared. Even without interventional bowel or bladder preparation, 25% of patients remained feasible to treat. The main limiting factors for treatment were disease beyond focal range of the transducer, and bone in the beam path. In n=3 patients, bone was a barrier because some of the lesion was too close to a bone surface; in n=9, bone could have been avoided by beam angulation, but this placed disease beyond reach of the transducer. If it were not possible to administer bladder or bowel preparations before treatment, up to a quarter of disease would have been considered non-targetable.Acknowledgements
CRUK and EPSRC support to the Cancer Imaging Centre at ICR and RMH in association with MRC & Department of Health C1060/A10334, C1060/A16464. NHS funding from the NIHR Research for Patient Benefit programme (PB-PG-0815-20001), and to the NIHR Biomedical Research Centre, the Clinical Research Facility in Imaging and the Cancer Research Network. We would also like to acknowledge the support of Philips Healthcare and the Focused Ultrasound Foundation.References
1. Dreyer, G., et al., Management of recurrent cervical cancer. Best Pract Res Clin Obstet Gynaecol, 2005. 19(4): p. 631-44.
2. Kim, Y.J., et al., Retrospective review of symptoms and palliative care interventions in women with advanced cervical cancer. Gynecol Oncol, 2015. 139(3): p. 553-8.
3. Ang, C., et al., Exenterative surgery for recurrent gynaecological malignancies. Cochrane Database Syst Rev, 2014(2): p. CD010449.
4. Chiva, L.M., et al., Surgical treatment of recurrent cervical cancer: state of the art and new achievements. Gynecol Oncol, 2008. 110(3 Suppl 2): p. S60-6.
5. Quinn, S.D. and W.M. Gedroyc, Thermal ablative treatment of uterine fibroids. Int J Hyperthermia, 2015. 31(3): p. 272-9.
6. Machtinger, R., et al., MRgFUS for pain relief as palliative treatment in recurrent cervical carcinoma: a case report. Gynecol Oncol, 2008. 108(1): p. 241-3.
7. https://clinicaltrials.gov/ct2/show/NCT02714621. MR-HIFU for Recurrent Gynaecological Cancer (HIFU-Gynae). 2017 [cited 2017 20/09/2017].
8. Huisman, M., et al., Feasibility of volumetric MRI-guided high intensity focused ultrasound (MR-HIFU) for painful bone metastases. J Ther Ultrasound, 2014. 2: p. 16.
9. Kim, Y.S., et al., MR thermometry analysis of sonication accuracy and safety margin of volumetric MR imaging-guided high-intensity focused ultrasound ablation of symptomatic uterine fibroids. Radiology, 2012. 265(2): p. 627-37.
10. Kim, Y.S., et al., Techniques to expand patient selection for MRI-guided high-intensity focused ultrasound ablation of uterine fibroids. AJR Am J Roentgenol, 2014. 202(2): p. 443-51.
11. Kim, Y.S., H.K. Lim, and H. Rhim, Magnetic Resonance Imaging-Guided High-Intensity Focused Ultrasound Ablation of Uterine Fibroids: Effect of Bowel Interposition on Procedure Feasibility and a Unique Bowel Displacement Technique. PLoS One, 2016. 11(5): p. e0155670.
12. McDannold, N., et al., Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology, 2006. 240(1): p. 263-72.
Figures