4046
Experimental demonstration of a HIFU self-scanning treatment in moving tissue using hybrid ultrasound and MR guidance1Radiology, University of Geneva, Faculty of medicine, Geneva, Switzerland, 2Biomedical Engineering, Center of Medical Image Analysis and Navigation, University of Basel, Basel, Switzerland, 3Radiology, Geneva Hospitals, Geneva, Switzerland
Synopsis
MRI-controlled tumor treatment by High Intensity Focused Ultrasound (HIFU) is challenging in the abdominal organs because of the breathing motion. We experimentally demonstrated a self-scanning method for motion compensation by passively scanning the tissue passing through the static focal point. Ex vivo turkey samples were subjected to a breathing-like non-periodic motion and the HIFU power was modulated using the velocity information from landmark tracking on simultaneous ultrasound images. MR imaging provided targeting and on-line thermometry. Temperature map were computed using the reference-less PRFS method. A dramatic improvement of the isotherms uniformity score was achieved for rectilinear volumetric ablation.
Introduction
During the last decade, High Intensity Focused Ultrasound (HIFU) treatment guided by Magnetic Resonance (MR) was widely developed and used for non-invasive thermal ablation of tumors. In addition to the intra-operatory planning, MR is used for on line thermometry and radiation force imaging. However, HIFU ablation remains challenging for abdominal organs undergoing respiratory motion. Current motion reduction techniques consist of apnea, gating1 or target tracking2 but treatments are time-consuming or require rapid switching capabilities of complex hardware. We are demonstrating experimentally a novel self-scanning paradigm consisting in maintaining the focal spot static and letting tissue pass through the HIFU beam thanks to its natural motion, generating a linear heating pattern. The objective is to exploit the breathing motion and the active power modulation of the HIFU beam to uniformly heat tumor tissues. The treatment control consists in computing on-the-fly the optimal HIFU power based on the detection and tracking of points of interest in the moving tissues as visualized by standard ultrasound images concurrently to motion compensated PRFS MR thermometry.Material and methods
Ex vivo turkey breast tissue sample (n=8) were subjected to a breathing-like non-periodic motion, generated by an MR-compatible robot (Innomotion) performing a back and forth straight-line movement of 15-20mm peak-to-peak amplitude. Focused ultrasound was generated by an MR-compatible multi-element phased array transducer (Imasonic, France) operating at 1MHz, with a focal length of 130 mm and powered by a 256-channel beam former (IGT, France). Ultrasound images used for the real-time tracking of ultrasound speckle were acquired by an MR-compatible linear array embedding 192 elements (Acuson Antares, Siemens Healthcare, Mountain View) at 30 fps. The ultrasound probe was placed in the coronal plane to visualize the sample motion (Figure 1). The calculation of motion vectors has a twofold interest: 1) the compensation and stabilization of the MR images using on-the-fly reprogramming of excitation and read-out pulse sequence blocks and 2) the modulation of the HIFU beam power in the range 0 – 150 W based on the tissue velocity magnitude. Experimental data were acquired in a whole body 3T MRI scanner (Prisma Fit, Siemens, Germany) by a segmented GRE-EPI sequence in the coronal plane through the focal spot using a 11 cm diameter receive loop coil. The temperature rise was computed by the reference-less PRFS temperature-sensitive method (resolution 1x1x3 mm3). MR images were reconstructed in real-time and magnitude data was merged with the temperature map by Thermoguide software (IGT, France). A thermal uniformity score along the self-scanning pattern was defined to evaluate the spatial uniformity of isotherms as the ratio between the temperature elevation at the mid-point and the average of the temperature elevation at the extremities.Results
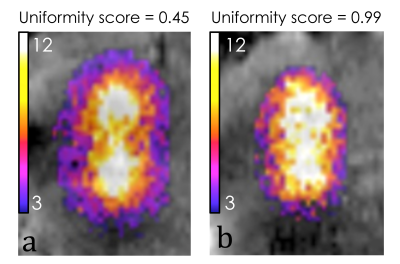
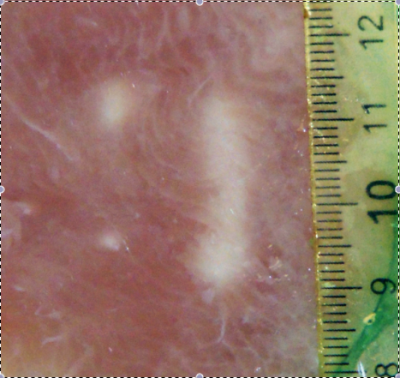
MR images were free of interferences during the simultanteous operation in-bore of ultrasound imaging, HIFU device and interventional robot. A flat power sonication scored 0.45 uniformity (Figure 2, a) corresponding to two distinct hot spots while modulated power sonication scored 0.99 uniformity (Figure 2, b) corresponding to a uniform lesion. Post-ablation gross pathology demonstrated a precise rectilinear pattern of tissue decoloration using active power modulation contrarily to a bi-focal pattern when applying a flat power (Figure 3). During the fast motion phase of the breathing cycle, the modulation algorithm applied up to 8 fold higher power to compare with the quiet phase.Discussion
Breathing is producing primarily rectilinear patterns, which in turn were demonstrated advantageous for rapid volumetric ablation while minimizing the thermal pattern drift downwards the near field3. Active motion tracking and focal spot locking on a target was demonstrated by various authors to be feasible4-7, however at a cost of complex software development and rapid switching capabilities of the phased array applicator. The self-scanning method reduces the hardware requirements, typically an annular transducer would be sufficient. This may help the clinical translation of the technology.
Conclusion
This study is the first experimental demonstration of a self-scanning treatment combining MR imaging and ultrasound imaging. It demonstrated a dramatic improvement of the spatial heating pattern despite changes in the respiratory motion (e.g. deep breath, cough) thanks to the power adaptation on-the-fly. This method also allows a shorter treatment time due to a 100% duty cycle and treats large volume of tumors without using electronic beam forming and without side lobe effects.Acknowledgements
No acknowledgement found.References
1. Auboiroux V, Petrusca L, Viallon M, Muller A, Terraz S, Breguet R, Montet X, Becker CD, Salomir R. Respiratory-gated MRgHIFU in upper abdomen using an MR-compatible in-bore digital camera. Biomed Res Int. 2014;2014:421726.
2. De Senneville BD, Mario Ries M, Bartels LW, and Moonen TW. Mri-guided high-intensity focused ultrasound sonication of liver and kidney. In Interventional Magnetic Resonance Imaging, pages 349–366. Springer, 2012.
3. Petrusca L, Auboiroux V, Goget T, Viallon M, Muller A, Gross P, Becker CD, Salomir R. A nonparametric temperature controller with nonlinear negative reaction for multi-point rapid MR-guided HIFU ablation. IEEE Trans Med Imaging. 2014 Jun;33(6):1324-37.
4. Celicanin Z, Manasseh G, Petrusca L, Scheffler K, Auboiroux V, Crowe LA, Hyacinthe JN, Natsuaki Y, Santini F, Becker CD, Terraz S, Bieri O, Salomir R. Hybrid ultrasound-MR guided HIFU treatment method with 3D motion compensation. Magn Reson Med. 2017 Sep 24. doi: 10.1002/mrm.26897.
5. Celicanin Z, Auboiroux V, Bieri O, Petrusca L, Santini F, Viallon M, Scheffler K, Salomir R. Real-time method for motion-compensated MR thermometry and MRgHIFU treatment in abdominal organs. Magn Reson Med. 2014 Oct;72(4):1087-95.
6. Holbrook AB, Ghanouni P, Santos JM, Dumoulin C, Medan Y, Pauly KB. Respiration based steering for high intensity focused ultrasound liver ablation. Magn Reson Med. 2014 Feb;71(2):797-806.
7. Ries M, de Senneville BD, Roujol S, Berber Y, Quesson B, Moonen C. Real-time 3D target tracking in MRI guided focused ultrasound ablations in moving tissues. Magn Reson Med. 2010 Dec;64(6):1704-1.
Figures