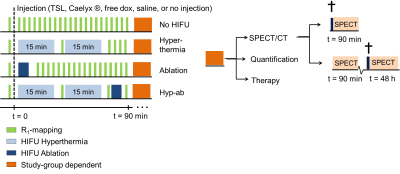4043
Synergistic effect of MR-HIFU hyperthermia mediated drug delivery followed by ablation1Oncology Solutions, Philips Research, Cologne, Germany, 2Biomedical NMR, Eindhoven University of Technology, Eindhoven, Netherlands, 3Oncology Solutions, Philips Research, Eindhoven, Netherlands, 4Department of Radiology, Experimental Imaging and Image-guided Therapy, University Hospital of Cologne, Cologne, Netherlands
Synopsis
Four Magnetic Resonance-guided High Intensity Focused Ultrasound (MR-HIFU) thermal therapy strategies (no HIFU, hyperthermia, ablation and hyperthermia followed by ablation) in combination with temperature sensitive liposomes (TSLs), co-encapsulating doxorubicin (dox) and ProHance®, were investigated in rhabdomyosarcoma rat tumor model. All HIFU heating strategies combined with TSLs resulted in cellular uptake of dox deep into the interstitial space and significant increase of intratumoral drug concentrations. The combination of hyperthermia-triggered TSLs followed by ablation showed the best therapeutic outcome compared to other strategies due to direct induction of thermal necrosis in the tumor core and efficient drug delivery to the tumor rim.
Purpose/introduction
Systemic chemotherapy is still the main therapeutic option for cancer treatment. Therapeutic efficacy is limited by off-target toxicity, leading to severe side effects for cancer survivors. Hyperthermia-triggered local drug delivery using doxorubicin-filled temperature sensitive liposomes (TSLs) has been shown to achieve 5-20 times (1-4) higher intratumoral drug concentrations. However, poorly perfused tumor areas remain undertreated and present a source for recurrence. Magnetic resonance-guided high intensity focused ultrasound (MR-HIFU) is a non-invasive heating technique that allows both prolonged hyperthermia for local drug delivery as well as ablation. We investigated different MR-HIFU treatment options comprising hyperthermia and ablation in combination with dox-TSLs with respect to intratumoral distribution of dox and liposomes, their overall biodistribution and therapeutic efficacy (3).Besides dox, also the MR contrast agent Prohance© was encapsulated in TSLs, allowing MR-based quantification and visualization for image guided drug delivery.Methods
TSLs composed of DPPC:HSPC:Chol:DPPE-PEG2000 (50:25:15:3 molar ratio) were prepared to encapsulating dox and ProHance®(5). For SPECT and histology, TSLs were labelled with 111Indium. The TSLs were loaded, either with a dox solution doped with 14C dox or cold dox. Dox concentration was determined by liquid scintillation counting. R1 rhabdomyosarcoma tumors were inoculated in the hind leg of female Wag/Rij rats (Charles River, age 5-7 weeks, N=113). Animals were enrolled into the study when tumor size was > 400 mm3. Under anaesthesia, the tumor bearing leg was depilated and analgesia was administered. The animal was positioned in a dedicated small animal MR-HIFU setup placed on the 3T Philips MR-HIFU Sonalleve tabletop (6). Respiration and body temperature were continuously monitored. Four MR-HIFU treatment arms were defined: 1) no HIFU, hyperthermia (4mm treatment cell, acoustic frequency f=1.44 MHz, acoustic power Pac= 10-15W, duration 2 times 15 min), ablation (4mm treatment cell, f=1.44 MHz, Pac= 35W, thermal dose > 240 CEM43°C (cumulative equivalent minutes at 43 °C)) and hyperthermia followed by ablation (see Figure 1). TSLs were injected just before sonication. The treatment cell was placed in the core of the tumor resulting a partial treatment of the lesion. Pre and post-treatment T1 maps were acquired using an inversion recovery Look-Locker sequence from which R1 maps were calculated (R1=1/T1). After treatment, animals were subjected to either SPECT imaging at 90 min or 48 hours post treatment, dox quantification or their tumor growth was monitored (see Table 1). Intratumoral dox quantification was compared to free dox injection. To compare the different therapy efficacies to standard of care, groups receiving saline (control), free dox and Caelyx® (DOXIL®) were added to the therapy study. Tumor size and body weight were measured at regular intervals and were evaluated for survival and toxicity, respectively.Results
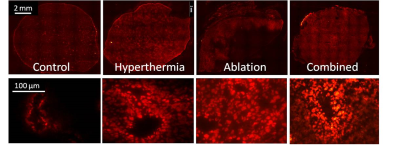
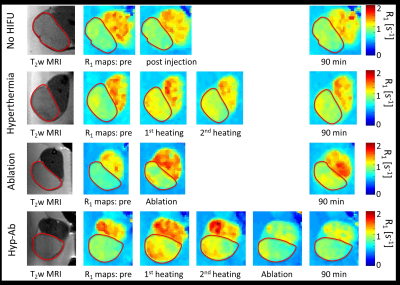
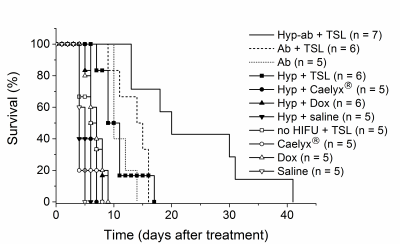
The intratumoral dox uptake in the no HIFU group (Control) is lower compared to the other thermal strategies (Figure 2). Hyperthermia+TSLs yields uptake in well perfused areas with high dox penetration into the interstitial space. For ablation, dox distribution is highest around the ablated area, however, with an intermediate zone of low dox uptake. The combination of hyperthermia and ablation leads to high dox uptake and non-viable areas due to thermal necrosis. Dox penetration depth was higher with HIFU compared to no HIFU treatment group, ~50 mm vs. ~10 mm. Figure 3 gives an overview of the different R1 maps during the different phases of the different treatment options. Only significant changes in tumor R1 were found: after the first hyperthermia period (15 min versus pre-treatment: ΔR1 = 0.12±0.09 s-1, p < 0.001) and after the ablation in the ablation group (ΔR1 = 0.44±0.31 s-1, p < 0.001). Also significant R1 changes were found in the muscle near the tumor in the ablation group (after ablation versus pre-treatment: ΔR1 = 0.14±0.16 s-1, p = 0.001). With respect to MR quantification of hyperthermia-triggered drug release, the pooled no HIFU and hyperthermia treatment groups showed a good correlation between intratumoral dox concentration and tumor average R1 change pre and 90 min post treatment (R2 = 0.43). The Kaplan-Meier analysis in Figure 4 shows that the survival (tumor reaching 3 times the per treatment size) for the ablation, ablation+TSL and hyperthermia+TSL is longer compared to no heated and hyperthermia without TSLs. The combination of hyperthermia followed by ablation including TSLs showed the highest therapeutic efficacy. No significant difference in toxicity was found.Conclusion
The unique MR-HIFU thermal treatment combination of hyperthermia followed by ablation showed clearly a synergistic effect on intratumoral dox concentration and survival. HIFU improved the penetration of dox into the tumor considerably. Dox release correlated well with observed tumoral R1 changes in the no HIFU and hyperthermia group.Acknowledgements
The authors thank Iris Verel (Philips Research Eindhoven), Caren van Kammen, Carlijn van Helvert, and Marleen Hendriks (all Maastricht University) for the support with the animal experiments. This research was performed within the framework of the Center for Translational Molecular Medicine (www.ctmm.nl), project VOLTA (grant 05T-201) and supported by NanoNextNL (FES0901:FES HTSM), the European Union project SONODRUGS (NMP4-LA-2008-213706) and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 603028 (iPaCT project).References
1. Partanen A, Yarmolenko PS, Viitala A, et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. International Journal of Hyperthermia [Internet] 2012;28:320–336. doi: 10.3109/02656736.2012.680173. 2. Staruch RM, Ganguly M, Tannock IF, Hynynen K, Chopra R. Enhanced drug delivery in rabbit VX2 tumours using thermosensitive liposomes and MRI-controlled focused ultrasound hyperthermia. International Journal of Hyperthermia [Internet] 2012;28:776–787. doi: 10.3109/02656736.2012.736670. 3. Hijnen N, Kneepkens E, de Smet M, Langereis S, Heijman E, Grüll H. Thermal combination therapies for local drug delivery by magnetic resonance-guided high-intensity focused ultrasound. Proceedings of the National Academy of Sciences 2017;114:E4802. doi: 10.1073/pnas.1700790114. 4. de Smet M, Heijman E, Langereis S, Hijnen NM, Grüll H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: an in vivo proof-of-concept study. 2010;150:102–10. doi: 10.1016/j.jconrel.2010.10.036. 5. de Smet M, Langereis S, van den Bosch S, Bitter K, Hijnen NM, Heijman E, Grüll H. SPECT/CT imaging of temperature-sensitive liposomes for MR-image guided drug delivery with high intensity focused ultrasound. Journal of Controlled Release 2013;169:82. doi: 10.1016/j.jconrel.2013.04.005. 6. Hijnen NM, Heijman E, Köhler MO, Ylihautala M, Ehnholm GJ, Simonetti AW, Grüll H. Tumour hyperthermia and ablation in rats using a clinical MR‐HIFU system equipped with a dedicated small animal set‐up. International Journal of Hyperthermia [Internet] 2012;28:141–155. doi: 10.3109/02656736.2011.648137.Figures