4042
Blood-brain barrier disruption and delivery of irinotecan in a rat model using a clinical transcranial MRI-guided focused ultrasound system1Radiology, Brigham and Women's Hospital, Boston, MA, United States
Synopsis
The feasibility of controlled blood-brain barrier (BBB) disruption in rats was demonstrated with a low-frequency clinical transcranial MRI-guided focused ultrasound device that operates at 230 kHz (ExAblate Neuro, InSightec) combined with microbubbles. Thirty-six targets were sonicated in one hemisphere in each experiment under closed-loop control based on real-time recordings of acoustic emissions. Disruption was confirmed in maps of R1 relaxation following Gadavist administration. After three weekly BBB disruptions covering an entire hemisphere, we always produced BBB disruption with only minor vascular side effects. We also delivered irinotecan chemotherapy across the BBB without apparent neurotoxicity.
Introduction
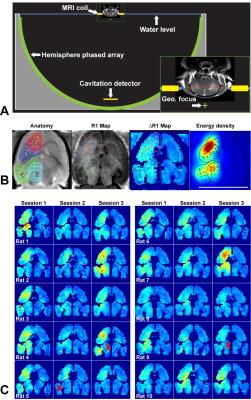
The use of ultrasound and intravenously-injected microbubbles is a promising noninvasive method to deliver drugs across the blood-brain barrier (BBB) that has been tested in numerous preclinical studies1 and early clinical trials2,3. For clinical translation, it is important to use the same device in animal studies that will be used clinically. However, the low frequencies used for transcranial sonication are challenging in small animal models, and large animal models are expensive. Here, we investigated the feasibility of controlled blood-brain barrier (BBB) disruption in rats using the ExAblate Neuro (InSightec, Haifa, Israel) low-frequency clinical transcranial MRI-guided focused ultrasound (FUS) device (Figure 1A). This device uses a hemispherical, 1024-element phased array transducer operating at 230 kHz that is integrated into a 3T MRI (Signa HDxt, GE Healthcare). We also tested the neurotoxicity of delivering the chemotherapy agent irinotecan to the brain.Methods
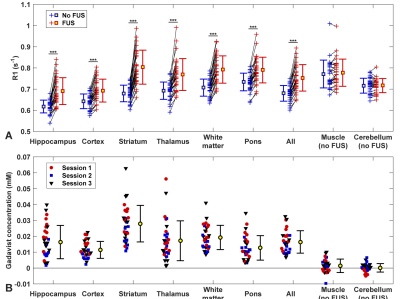
Animals received three weekly sessions of FUS alone, FUS and 10 mg/kg irinotecan, or irinotecan alone (N=5/group). In each session, four volumetric sonications (5 ms bursts applied at 1.1 Hz for 55 s) targeted 36 overlapping locations in one hemisphere (Figure 1B). Each volumetric sonication was combined with Definity microbubbles (10 µl/kg, Lantheus). Closed-loop feedback control over the acoustic power was performed based on recordings of acoustic emissions. BBB disruption was evaluated using maps of R1 relaxation following IV injection of 0.125 mmol/kg Gadavist (Bayer).Results
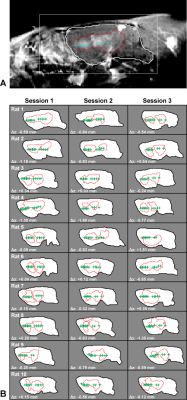
BBB disruption, evaluated by mapping the R1 relaxation rate after administration of an MRI contrast agent, was significantly higher in the sonicated hemisphere (P<0.01; Figure 1C). While we found substantial variations in the delivery of the contrast agent in different tissue structures (Figure 2), disruption was achieved in every session. The BBB disruption was constrained to a central axial depth within the brain (Figure 3); its length along the direction of the FUS beam was 3.5±0.7 mm. The mean absolute difference between the center of the disrupted region and the planned depth was 0.6±0.5 mm. This targeting error in this direction ranged from -1.9 to 1.5 mm and was less than 1 mm in 25/30 sessions.
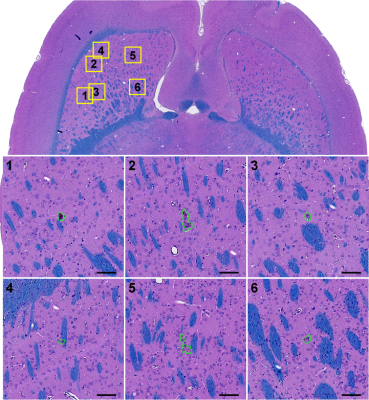
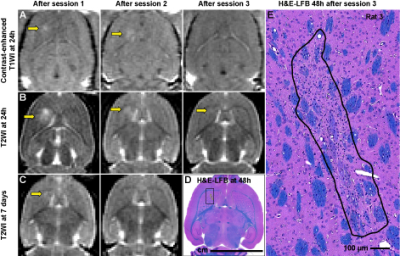
BBB disruption and edema in the striatum were evident in some cases at 24h after FUS (Figure 5A-C). Histological evaluation found minute (dimensions: 61.4±46.7 µm) clusters of extravasated erythrocytes or hemosiderin particles (Figure 4). In four animals, a tiny (0.5-1.2 mm) scar was found in the striatum (Figure 5D-E). Three of these cases were evident as hyperintense regions in T2-weighted MRI (B). Simulation of the acoustic field demonstrated that the acoustic energy density applied over all sonications was highest in the striatum, suggesting that our target spacing (1 mm) was perhaps too dense in that region (Figure 1B).
With feedback control 98% of the sonication targets (1045/1071) reached a pre-defined level of acoustic emission at the second and third harmonic of the 230 kHz FUS frequency, while the probability of wideband emission (a signature for inertial cavitation) was than 1%.
There were no significant differences in the acoustic exposure levels, acoustic emissions, or in the resulting BBB disruption or in the number of erythrocyte/hemosiderin clusters between animals that received FUS alone or FUS and irinotecan (P>0.05), and no difference in health or weight gain were observed between these animals and those that received drug alone. However, the dimensions of the petechiae/erythrocyte clusters were larger (P<0.001) in the animals that received FUS and irinotecan.
Discussion
It is possible to use a low-frequency clinical TcMRgFUS device for BBB disruption studies in rats. Actively controlling the exposure level until a significant increase in harmonic emissions compared to sonication without microbubbles was effective for ensuring that BBB disruption occurs with minimal vascular damage. Overzealous overlap of sonication targets might lead to edema, prolonged BBB disruption, and tissue damage.
Irinotecan delivery to the healthy brain does not appear to be neurotoxic. If we can safely deliver this chemotherapy agent to the margins of a brain tumor (where the BBB is intact) we may be able treat infiltrating tumor cells that often lead to recurrence. Irinotecan has been evaluated in several clinical trials in glioma patients4,5, and these results are promising for evaluating its use with FUS-induced BBB disruption.
Acknowledgements
This work was supported by InSightec, the Focused Ultrasound Foundation, and by NIH grant P01CA17464501. Use of the ExAblate Neuro system for this work was provided by InSightec.References
1. K.Hynynen, N.McDannold, N.Vykhodtseva, and F.A.Jolesz, "Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits," Radiology 220(3), 640-46 (2001).
2. A.Carpentier, M.Canney, A.Vignot, V.Reina, K.Beccaria, C.Horodyckid, C.Karachi, D.Leclerq, C.Lafon, J.Y.Chapelon, L.Capelle, P.Cornu, M.Sanson, K.Hoang-Xuan, J.Y.Delattre, and A.Idbaih, "Clinical trial of blood-brain barrier disruption by pulsed ultrasound," Sci.Transl.Med. 8(343), 1-9 (2016).
3. Y.Huang, R.Alkins, M.Chapman, J.Perry, A.Sahgal, M.Trudeau, T.G.Mainprize, and K.Hynynen. "Initial Experience in a Pilot Study of Blood-Brain Barrier Opening for Chemo-Drug Delivery to Brain Tumors by MR-Guided Focused Ultrasound." in: Proc.24th Meeting of the ISMRM, Singapore. 450. (2016)
4. J.J.Vredenburgh, A.Desjardins, D.A.Reardon, and H.S.Friedman, "Experience with irinotecan for the treatment of malignant glioma," Neuro.Oncol. 11(1), 80-91 (2009).
5. J.N.Jakobsen, B.Hasselbalch, M.T.Stockhausen, U.Lassen, and H.S.Poulsen, "Irinotecan and bevacizumab in recurrent glioblastoma multiforme," Expert.Opin.Pharmacother. 12(5), 825-33 (2011).
Figures