4040
Design of Focused Radio Frequency Heating Array Coils for Non-Invasive Hyperthermia with Ultra-High Field MRI1Department of Radiology, Stanford University, Stanford, CA, United States, 2Radiological Sciences Laboratory, Stanford, CA, United States
Synopsis
This abstract details the implementation of an automated pipeline for designing high channel count RF coils for the purpose of non-invasive Focused RF hyperthermia generated from Ultra-high field MRI parallel transmit coils. The pipeline integrates multiple tools including Sim4Life for EM-FDTD simulations in the Virtual Population physiologically realistic body models, Advanced Design Systems circuit simulator, and Matlab for custom algorithm execution. This work leverages GPU acceleration which has reduced simulation times for an 84 channel RF coil from 77 days on a CPU to 6 hours on three compute nodes equipped with 14 affordable GPUs.
INTRODUCTION
The concern about increased specific absorption rate (SAR) of power deposition at ultra high field (UHF) has given rise to novel algorithms1 that leverage the increased degrees of freedom of parallel transmit RF array coils to minimize SAR deposition. This abstract presents work aimed at achieving the opposite: delivering focal maximum SAR to an arbitrarily specified target volume for the purpose of intentionally causing tissue heating for therapeutic purposes. The goal is to accomplish non-invasive thermal therapy using commonly available UHF MRI technology; applications could include focal opening of the blood-brain barrier via hyperthermia2,3 and activation of temperature-sensitive therapeutic nanoconstructs. We refer to this concept as MR-guided Focused RF (MRgFRF). The optimal design of the Focused RF (FRF) transmit array remains to be determined, but simulating even a single FRF array design is a tedious process. To quantify the effects of design parameters such as operating frequency, number of required array elements, etc, large numbers of design simulations will be required. No single existing tool can perform all the evaluations required. We have developed a software toolchain which automates the interaction of several simulation tools and exploits GPU acceleration to efficiently perform electromagnetic and thermal simulations in realistic computational human body models4. This toolchain is founded on the Sim4Life (SPEAG, Zurich) multi-physics modeling package, interfaced with the Advanced Design Systems (Keysight, Santa Rosa, USA) circuit simulator, and the Virtual Population4 physiologically realistic body models (IT’IS, Zurich).METHODS
We have previously presented maxSAR5: an efficient algorithm that maximally focuses RF energy into a target volume. In this abstract, we have implemented a comprehensive and highly automated FRF array design pipeline (Figure 1) around this algorithm to study array coil designs with 8-128 channels. Figure 2 shows the framework’s flexibility in specifying coil element geometry. Electromagnetic (EM) simulations are then run for tuning the coil to a desired frequency. Since lumped element circuit components are unspecified at this stage, one simulation is run for each port where it is the active voltage input port and all other ports are 50 Ω loads. The resulting S-matrix relates how each port responds when another port is excited. Figure 3 illustrates an 84 channel elliptical design. The toolchain automates 420 simulations, producing results quickly and consistently. The EM simulations were performed on NVIDIA 1080Ti GPUs using the Virtual Population “Ella” human model. A batch processor takes the S-matrices and runs a circuit optimization routine developed in ADS to solve for the capacitor values that tune the coil to the desired frequency. The toolchain automates the process of importing these values into the Sim4Life coil description. Final EM field maps for each channel are then generated in Sim4Life. These field maps are exported to Matlab where the maxSAR algorithm uses them to maximizes SAR (and consequently tissue heating) in a target region. The resulting total volumetric SAR which can be used with the Sim4Life thermal solver to compute the heating achievable by each design.RESULTS
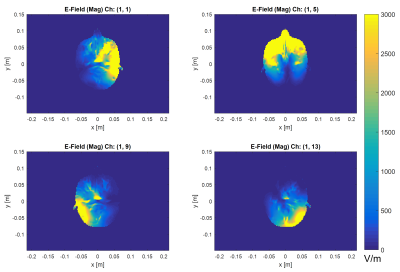
The tune/match pipeline step (see Figure 1) took approximately 30 minutes per coil element using 2x1080 Ti GPU accelerators. Using the 14 GPUs available in our compute nodes, 7 coil element simulations were processed in parallel. In total, 6 hours of computation time were required for all 84 channels. Representative electric field maps are shown in Figure 4 for four coil elements of the 84 channel elliptical FRF coil design.DISCUSSION
The design pipeline described will make it possible to more quickly and efficiently optimize the FRF system design. We envision two possible hardware configurations for FRF hyperthermia: (1) a dedicated applicator as shown in figure 3, and (2) an “all-in-one” approach where the imaging RF coil is also used for FRF heating. The all-in-one approach would be elegant, potentially making any MRI system into a therapeutic modality. UHF MRI scanners commonly have 8 parallel transmit RF amplifiers, although systems with 16 and even 32 transmit channels are available, which should provide sufficient degrees of freedom to accomplish FRF. However, the dedicated applicator approach affords more design flexibility (e.g. separate optimization of RF coils for imaging and heating), and would allow simultaneous operation of the FRF heating and imaging RF coils at independent frequencies. The pipeline we introduce here will enable us to quantify these tradeoffs in future work.CONCLUSION
We have developed an efficient coil design pipeline that has greatly accelerated the design of high-channel-count focused RF heating coils. Focused RF heating has the potential to transform UHF MRI into a combined therapeutic and diagnostic modality capable of non-invasive hyperthermia.Acknowledgements
The authors would like to acknowledge research support by GE Healthcare and by NIH T32 CA009695, NIH P41 EB015891 and NIH 1 U01 EB025144-01.References
REFERENCES:
1. Pendse M, Rutt BK. Overcoming limitations of virtual observation points in ptx using impulse. In: Proceedings of the ISMRM. Vol 25, 2017.
2. Shivers RR, Wijsman JA. Chapter 19 blood-brain barrier permeability during hyperthermia. In: Progress in Brain Research. Vol 115. Elsevier; 1998:413-424.
3. Moriyama E, Salcman M, Broadwell RD. Blood-brain barrier alteration after microwave-induced hyperthermia is purely a thermal effect: i. temperature and power measurements. Surg Neurol. 1991;35(3):177-182.
4. Gosselin M-C, Neufeld E, Moser H, et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: the virtual population 3.0. Phys Med Biol. 2014;59(18):5287-5303.
5. Pendse M, Rutt BK. An algorithm for maximum-sar targeted RF hyperthermia. In: Proceedings of the ISMRM. Vol 23. ; 2015:3224.
Figures