4039
Early clinical feasibility study of body mounted and motorized needle guidance tool in abdominal MRI-guided cryoablation1The Ferenc Jolesz National Center for Image Guided Therapy, Radiology, Boston, MA, United States, 2Healthcare Optics Research Laboratory, Canon USA, Inc., Cambridge, MA, United States
Synopsis
This study investigates the feasibility of directing cryotherapy probes using a body-mounted and motorized needle guidance device in abdominal MRI-guided cryoablation. Utilizing a motorized needle alignment device offers an alternative solution to the trial-and-error method of probe placement, as it allows one to directly orient cryotherapy probes, efficiently. This study found the method to be feasible for use in cryoablation in 3T with placement accuracy of 8.8mm. Further investigation is warranted to improve the efficiency of probe placement.
INTRODUCTION
MRI-guided cryoablation is a routine procedure for diagnosing and treating masses in the abdomen, pelvis, and chest1. During these procedures, applicator probes are inserted percutaneously into a patient’s body to freeze the masses. In the current practice, these probe tools are entered using a trial-and-error fashion, until the desired trajectory is established. There are two disadvantages to the trial-and-error process: (1)repeated trials can increase the procedure time, and (2) repeated trials can increase the risk of bleeding and injury to adjacent organs. Our goal is to achieve an ideal placement of the cryotherapy probes using a method that will avoid the trial-and-error process. Our proposed solution utilizes a motorized needle alignment that directs the tools stereotactically. This method may facilitate the accurate placement of the probes and allows the formation of an ablation zone that sufficiently covers the mass without damaging the surrounding tissue. The objective of the study, therefore, is to collect preliminary data to investigate the feasibility of using guiding cryotherapy probes and motorized needle alignment tools in MRI-guided cryotherapy.METHODS
All subjects will be patients requiring an MRI-guided percutaneous cryoablation procedure in the abdomen, pelvis or chest using a wide-bore 3T MRI scanner (MAGNETOM Verio 3T, Siemens, Erlangen, Germany). A a body-mounted, motorized needle alignment device that can maintain the geometrical relationship between the device and the patient’s body, even in the presence of the patient’s frequent body motions, was clinically tested in this study after the Institutional Approval Board’s clearance was obtained for the device2. The device is comprised of two ring-shaped rotary stages stacked on each other, in which the top rotary stage holds the cryotherapy probe. The rotary stages can orient the cryotherapy probe with the skin incision point as the remote-center-of-motion. In the approved protocol, the device orient the probe to aim the lesion defined in the intra-procedural T2 images. The protocol however didn’t include the actual insertion of the probe using the device. To place the device on a loop body coil, as well as a modular surface coil, a jig mount was developed to attach the device2.RESULTS
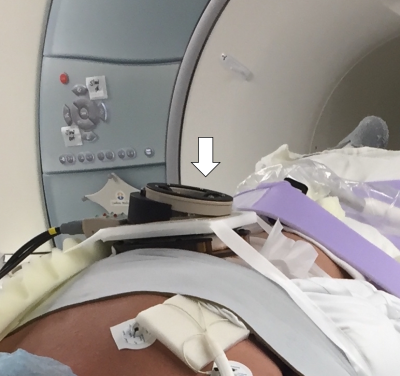
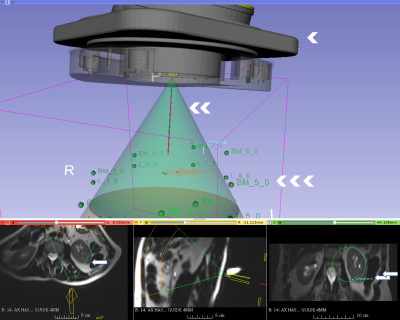
Ten patients agreed to enroll in the study between October 2016 and January 2017. The study included MRI-guided cryoablation of kidney (five cases), liver (one case), retroperitoneal (three) and diaphragmatic (one). In two cases, mechanical issues have forced an early termination of the use of the device. In one case, the size of the patient caused the device to be at the periphery of the imaging volume where there is distortion, causing erroneous image-to-device registration. In the other seven successful cases aiming total of 15 targets, we have confirmed that we can (1) place the device on the patient (Figure 1), (2) perform MR imaging to register the location of the device, (3) aim probes guide to targets, (4) acquire another set of MR images to assess the trajectory of the guide (Figure 2). The error to aim 15 targets at an average of 112.3 mm away from the device was 8.8 +/- 4.9 mm with the range of 3.1 mm to 19.9 mm. The entire device, itself, moved an average of 1.3 mm in translation and 0.84 degrees in rotation between the pre-targeting and post-targeting scans. The average error of targeting to lesion at 100 mm depth or less is 6.5 mm, and the average error to targets 132 mm or higher is 12.3 mm. There were no targets at depths between 100 and 132 mm. No adverse clinical event occurred due to the use of the device.DISCUSSION
We have successfully performed clinical deployment of the body-mounted, motorized needle alignment device in MRI-guided cryotherapy. Our study found that the device can orient the cryoablation probe with accuracies in the range of 3.08 mm to 19.87mm. The accuracy of the probe placement was not compared against the manual approach, or the size of the lesion targeted. At the same time, the accuracy of 8.8 mm seems to be relatively close to the 6.6 mm of placement accuracy we gained in phantom studies2. The results of this study provide the foundation and accountability needed to perform future clinical trials that include the insertion of a probe using a motorized needle alignment device, as a guide. This trial also supports the hypothesis that no adverse effects arise from the operation of this needle guidance device, and it is our contention that it will provide the groundwork for comparing the accuracy of guided trajectories against guideless trajectories. A body-mounted and motorized needle alignment device can be safely used in MRI-guided cryotherapy.Acknowledgements
Canon USA Inc and NIH/NIBIB P41-EB015898References
1. Ahrar, K., Ahrar, J. U., Javadi, S., Pan, L., Milton, D. R., Wood, C. G., … Stafford, R. J. (2013). Real-Time Magnetic Resonance Imaging–Guided Cryoablation of Small Renal Tumors at 1.5 T. Investigative Radiology, 48(6), 437–444. https://doi.org/10.1097/RLI.0b013e31828027c2
2. Hata, N., Song, S.-E., Olubiyi, O., Arimitsu, Y., Fujimoto, K., Kato, T., … Tokuda, J. (2016). Body-mounted robotic instrument guide for image-guided cryotherapy of renal cancer. Medical Physics, 43(2), 843–853. https://doi.org/10.1118/1.4939875
Figures