4026
MRS-based metabolomic approaches detect multiple metabolic targets for the antitumor action of metformin on U87MG glioma cells.1Istituto Superiore di Sanità, Roma, Italy, 2IRCCS Istituto Oncologico Veneto, Padova, Italy
Synopsis
There is an urgent need to develop novel therapeutic strategies for malignant brain tumors. Purposes of this study were: 1) to investigate in glioma cells the alterations induced by metformin in the levels of metabolites produced by different biochemical pathways ( glycolysis and phosphatidylcholine (PC) metabolism); 2) to evaluate the possibility to enhance the antiproliferative effects of metformin by a combination of metformin with a selected inhibitor of PC metabolism. We found : a) activation of PC-plc and PCho accumulation in metformin-treated cells; b) enhanced cell death in glioma cells with a combination of metformin and the PC-plc.
Introduction: Glioblastoma multiforme (GBM), the most malignant among brain tumors are refractory to conventional treatments including surgery, chemotherapy, and radiotherapy. This aggressiveness and malignancy justifies and draws attention to the urgent need to develop novel therapeutic strategies. The anti-diabetic drug metformin exhibits anti-proliferative effects in preclinical models of human GBM, although the underlying molecular mechanisms are unclear. It is known that metformin activates adenosine monophosphate-activated protein kinase (AMPK), a sensor of cellular energy, and inhibit AKT activity.1,2
Purposes of this study were: 1) to investigate in human U87MG glioma cells the concomitant alterations induced by metformin in the levels of metabolites produced by different biochemical pathways such as glycolysis and phosphatidylcholine (PC) metabolism; 2) to evaluate the possibility to enhance the antiproliferative effects of metformin by a suitable combination of this agent with a selected inhibitor of PC metabolism.
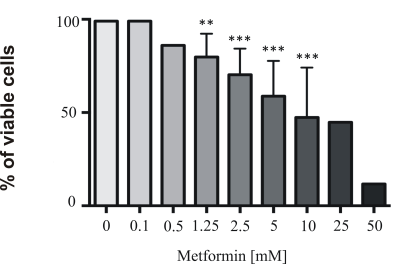
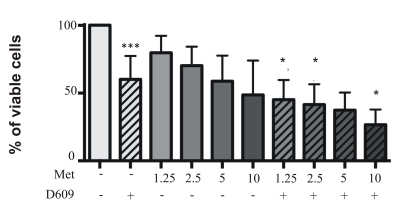
Material and methods: Metabolomics analyses were performed using magnetic resonance spectroscopy (MRS) on aqueous cell extracts of either untreated (control) or metformin-treated-human glioma cells U87MG cells. Since in a previous our work we observed that PC-plc inhibition interferes with proliferation, invasion and glycolysis in U87MG glioma cells,3 we further exposed these cells to D609, the competitive inhibitor of PC- specific phospholipase C (PC-plc), an enzyme known for its implications in cell signaling, cell cycle regulation and cell proliferation.4,5 U87MG cells were exposed to 100µM D609 in combination with increasing doses of metformin (from 0.1 mM to 10 mM) for 72 h and cell viability was assessed using the MTT-proliferation assay.
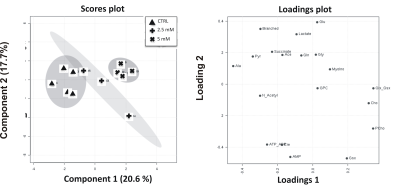
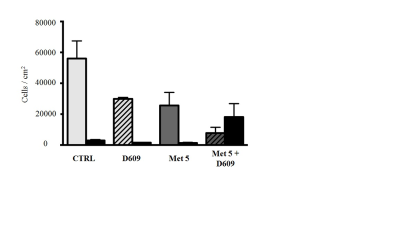
Results: Metformin exposure induced cell growth arrest but not cell death in U87MG cells (Figure 1). Multivariate analysis (PLS-DA) of intracellular aqueous low-weight metabolites of U87MG exposed for 72 h to metformin (2.5 and 5 mM) showed a clear segregation between metformin-treated and control cells (Figure 2). The main metabolites contributing to this segregation were alanine (ala) and phosphocholine (PCho), respectively decreasing and increasing in metformin exposed cells relative to control. PCho is a metabolite involved in both PC-biosynthesis by choline kinase and degradation by PC-plc4,5 .Under exposure to both metformin and D609, we found a reduction in PCho signal associated to a significant decrease of cell viability (Figure 3) and increased cell death (Figure 4, black columns). .
Conclusion: We provided evidence to support : a) activation of PC-plc and PCho accumulation in metformin-treated cells; b) induction of cell death in U87MG cells simultaneously with a combination of metformin and the PC-plc inhibitor as compared to alone metformin. These results provide the grounds for the possible development of a new multi-targeted approach against this malignancy.
Acknowledgements
This work was funded by grants from: Associazione Italiana per la Ricerca sul Cancro (AIRC) 2007–2010; Special Program Alleanza Contro il Cancro 2006, ACC3-AC9 Ministry of Health, Italy; Programma Oncotecnologico ISS/13ONC/5.
References
1) Sato A, Sunayama J, Okada M, et al. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem cells translational medicine. 2012;1:811–824.
2) Wurth R, Pattarozzi A, Gatti M, et al. Metformin selectively affects human glioblastoma tumor-initiating cell viability: A role for metformin-induced inhibition of Akt. 2013;12(1):145-56.
3) Mercurio L, Cecchetti S, Ricci A, et al. Phosphatidylcholine-specific phospholipase C inhibition down- regulates CXCR4 expression and interferes with proliferation, invasion and glycolysis in glioma cells. PLoS One. 2017;12(4):e0176108.
4) Podo F, Paris L, Cecchetti S, et al.. Activation of phosphatidylcholine-specific phospholipase C in breast and ovarian cancer: Impact on MRS-detected choline metabolic profile and perspectives for targeted therapy. Front Oncol (2016) 6:171.
5) Podo F, Canevari S, Canese R, et al. NMR Biomed. 2011 (6):648-72.
Figures