4020
Neurofunctional deficits and cellular metabolic changes associated with working memory dysfunction in patients with generalized anxiety disorderChung-Man Moon1 and Gwang-Woo Jeong2
1Advanced Institute of Aging Science, Chonnam National University, Gwangju, Republic of Korea, 2Radiology, Chonnam National University Medical School, Gwangju, Republic of Korea
Synopsis
Generalized anxiety disorder (GAD) negatively affects emotional regulation and cognitive function. However, the causality between neurofunctional and metabolic brain alterations is not clearly revealed since the brain function and metabolic studies have been independently performed. A combined fMRI and 1H-MRS study would be necessary to investigate whether both of the brain functional abnormality and the metabolic changes are associated with the emotional dysregulation and cognitive deficit in GAD. Therefore, the purpose of this study was to assess the association between neurofunctional deficits and cellular metabolic changes under working memory (WM) tasks in patients with GAD.
Introduction
Generalized anxiety disorder (GAD) negatively affects emotional regulation and cognitive function. However, the causality between neurofunctional and metabolic brain alterations is not clearly revealed since the brain function and metabolic studies have been independently performed. A combined fMRI and 1H-MRS study would be necessary to investigate whether both of the brain functional abnormality and the metabolic changes are associated with the emotional dysregulation and cognitive deficit in GAD. Therefore, the purpose of this study was to assess the association between neurofunctional deficits and cellular metabolic changes under working memory (WM) tasks in patients with GAD.Methods
The 28 right-handed subjects included 14 patients (mean age, 36.6±8.8 years), who had been diagnosed with GAD with mild levels of depression by a psychiatrist using the DSM-IV-TR and 14 age-matched healthy controls (mean age, 37.8±7.8 years). The duration of the patients′ illness was 3.2±3.5 years. Subjects underwent fMRI and 1H-MRS in WM task with emotion-inducing distractors at a 3 T Magnetom Verio MR Scanner (Siemens Medical Solutions, Erlangen, Germany) with a birdcage head coil.Results and Discussion
In response to the emotional distractors in WM tasks, the patients concurrently showed higher activity in the hippocampus and lower activities in the superior occipital gyrus, superior parietal gyrus, dorsolateral prefrontal cortex (DLPFC) and precentral gyrus compared to healthy controls (Fig. 1). In MRS, the ratio of choline/creatine (Cho/Cr) in the DLPFC, which is an important area involving the executive functions such as WM and cognitive flexibility, was significantly lower in the patients (0.72±0.10) than in the controls (0.81±0.08) (p=0.029) (Figs. 2 and 3). In particular, the Cho ratios were positively correlated with the brain activities based on blood oxygenation level-dependent (BOLD) signal change in the DLPFC (γ=0.843, p<0.0001) (Fig. 4). In this study, one of the major findings is the reduced BOLD signal in the DLPFC accompanied by a reduction of Cho concentration in patients with GAD. The DLPFC is a key center involved in cognitive functions including WM processing, attention and integration of emotion. It is assumed that decreased activation of the DLPFC by the emotion-inducing distractors in patients with GAD is associated with lower cognitive function and attention caused by emotional dysregulation compared to healthy controls. As for the metabolic changes in GAD, Cho indicates the level of acetylcholine, which is a neurotransmitter involved in the functions of attention, learning, and memory. Therefore, the level of Cho is potentially important for assessing the cognitive impairments including memory deficits and low attention in GAD. Also, presently Cho concentration in the DLPFC was positively correlated with the BOLD signal change under emotion-inducing distractors in GAD. In other words, the activity of the DLPFC in GAD is closely related to the level of Cho, which is associated with the pathological anxiety severity and cognitive dysfunction.Conclusion
This study provides the first evidence for the association between the metabolic alterations and neurofunctional deficit in WM processing with emotion-inducing distractors in GAD. These findings will be helpful to understand the neural dysfunction in connection with WM impairment in GAD.Acknowledgements
This work was supported by the fund from the National Research Foundation of Korea (2015R1A2A2A01007827; 2017R1A6A3A11030092).References
Price RB, Eldreth DA, Mohlman J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation. Transl. Psychiatry 2011;1:e46.Figures
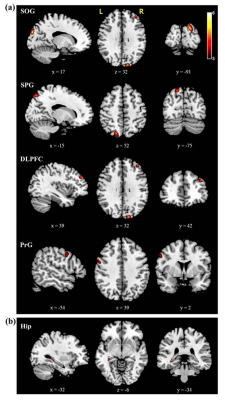
Figure 1. Brain areas predominantly activated in
healthy controls over patients with GAD (a) and GAD over healthy controls (b)
in the working memory tasks with the emotional distractors, which were resulted
from two-sample t-test (p<0.0005). SOG: superior occipital
gyrus; SPG: superior parietal gyrus; DLPFC: dorsolateral prefrontal cortex;
PrG: precentral gyrus; HIP: hippocampus. L=left; R=right.
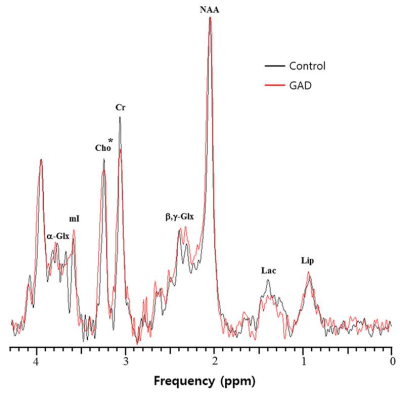
Figure 2. Representative
spectra of the DLPFC, which were acquired from a GAD patient (red) and a healthy control
(black). * indicates the metabolite with significantly different concentrations
between two groups (p<0.05).
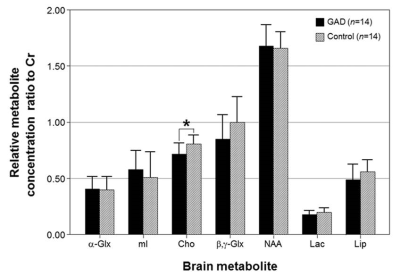
Figure 3. Relative metabolite concentration ratio to Cr
in the DLPFC in patients with GAD and healthy controls. * indicates the
significantly different metabolite concentrations between patients with GAD and
healthy controls (Mann-Whitney U test, p<0.05).
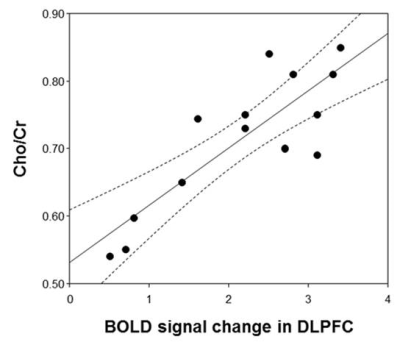
Figure 4. The
correlations of BOLD signal change in the DLPFC with Cho/Cr (γ=0.843, p<0.0001) in patients with GAD where
the band with dotted lines shows 95% confidence intervals.