4001
Detection of 5-Fluorouracil (5-FU) Tumor Trapping Using Fluorine-19 Chemical Shift Imaging in a Murine Model of Colorectal Cancer1Chemistry, Lakehead University, Thunder Bay, ON, Canada, 2Thunder Bay Regional Health Research Institute, Thunder Bay, ON, Canada, 3Medical Biophysics, University of Western Ontario, London, ON, Canada, 4Northern Ontario School of Medicine, Thunder Bay, ON, Canada, 5Thunder Bay Regional Health Science Centre, Thunder Bay, ON, Canada
Synopsis
Colorectal cancer is an increasing healthcare problem and is the third leading cause of cancer deaths worldwide. Fluorine-19 (19F) chemical shift imaging (CSI) can detect the distribution of 5-fluorouracil (5-FU) and its metabolites within a tumor over time. By measuring the 5-FU trapping, it is possible to determine the tumor response at the earliest stage of treatment (before changes in tumor size are observable). In this work, we present preliminary results of detecting the presence and absence of 5-FU trapping in mice bearing H-508 and HT-29 colorectal tumors, respectively.
Purpose
Colorectal cancer is the third most common cause of cancer deaths worldwide according to World Healthcare Organization[1]. 5-Fluorouracil (5-FU) is one of the most widely used cytotoxic drugs for treating a variety of solid cancers, including colorectal cancer[2,3]. However, 5-FU chemotherapy has a low efficacy[2,4] which can only be detected after chemotherapy has been completed. Therefore, a method to detect the resistance of tumors to 5-FU in the earliest stages of treatment is needed. 5-FU produces an MRI signal due to a single fluorine-19 (19F) atom in the molecule. Additionally, 5-FU catabolises into fluorinated compounds which can be detected using 19F MRI[5-7]. It has been demonstrated clinically that the “trapping phenomenon” (retention of 5-FU in the tumor longer than 20 minutes) correlates with positive tumor response to chemotherapy[8]. To detect the 5-FU signals, chemical shift imaging (CSI) was used due to the high sensitivity of this technique and its ability to detect 5-FU metabolites at different resonance frequencies. The purpose of this study was to identify if there was a detectable difference between the 5-FU signal-to-noise ratio (SNR) obtained from two different types of human colorectal cancer, one responsive and one non-responsive to 5-FU chemotherapies. Significant differences between the SNR and MR time courses allow us to infer the potential suitability of 19F CSI to detect the resistance of colorectal cancer to 5-FU treatment.Materials and Methods
Two different types of human colorectal cancer cells, HT-29 (known to be insensitive to 5-FU) and H-508 (known to be 5-FU responsive), were grown in a culture. A total of 23 anatomical nude mice grew either a HT-29 tumor (n=9), H-508 tumor (n=9), or both tumor types (n=5). For the latter, HT-29 and H-508 cells were injected into the left and right flanks respectively of the animals which were anesthetized using 2% isoflurane. The mice grew the tumors until they became perceptible. Before MR imaging, the mice were induced using 5% isoflurane, then 2% isoflurane was used to maintain the anesthesia during the experiment. All animals received a bolus tail vein injection of 300µL of 5-FU (50mg/mL). The mice were then imaged using a Clinical 3T Philips Achieva MRI scanner equipped with a 51 mm dual-tuned quadrature 1H/19F birdcage coil. A coronal T1-weighted 1H Fast Spin Echo multi-slice sequence was used for tumor localization. Proton scans were acquired using a FOV of 75x75mm2, resolution of 256x256, TR/TE=5000/55.19ms, slice thickness of 2 mm and NSA=3. 19F CSI images were acquired after the 5-FU bolus during a time interval up to 70 minutes, with a temporal resolution of 2.5 min. Mice with a single tumor type were imaged using CSI with 8×5 resolution, FOV=20×50mm2, and NSA=3, whereas mice with both tumor types were studied using 3×5 matrix, FOV=31×18.6mm2, and NSA=9. All CSI images were acquired using the spectral bandwidth of 32 kHz, TR/TE=5000/4.27ms.Results
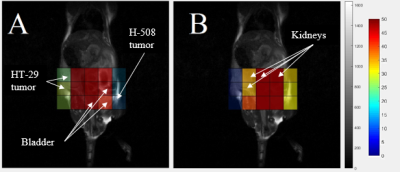
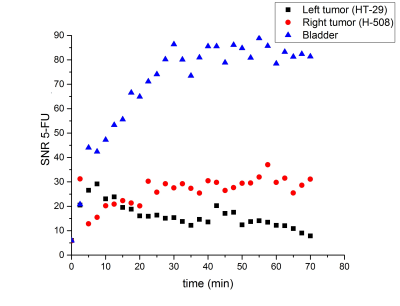
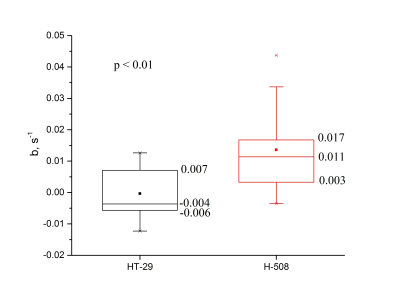
Representative 19F CSI images acquired from the animal with both tumors overlaid on the proton localizers are shown in Fig.1. The 5-FU SNR time dependencies are presented in Fig.2. All tumor time courses were fitted using the exponential function SNR=aebt. Amplitude and time constant were the fitting parameters. A two-sample Student test was applied to the time constant (Fig.3). The mean value of the time constant for H-508 tumor was significantly statistically larger than zero (b=0.014s-1; p<0.01), indicating positive 5-FU trapping, whereas for the HT-29 tumor it was slightly less than zero, indicating the resistance to 5-FU (b=-4x10-4s-1; p<0.01). The difference between the time constants for HT-29 tumor and H-508 tumor was statistically significant (p<0.01).
Discussion
The signal from the H-508 tumor in the mouse with both tumor types increased steadily throughout 22.5 minutes after the bolus injection of 5-FU, which leveled off and slightly oscillated around the mean value of 30 (Fig.2). The HT-29 signal increased significantly during the first 7.5 min and then decreased persistently throughout the remaining time. The mean value of the time constant being larger than zero indicates the presence of the trapping phenomenon in the H-508 tumor. On the contrary, the b value of less than zero shows the absence of trapping in the HT-29 tumor.We were not able to observe a signal from any of the 5-FU metabolites, possibly due to the low concentration of fluorinated metabolites in the organs.
Conclusion
Overall, this study provided proof of principle that 19F CSI can be used to detect the resistivity of different types of colorectal cancer to 5-FU chemotherapy based on the presence or absence of tumor trapping.Acknowledgements
This work was supported by a grant from the Northern Ontario Academic Medicine Association (NOAMA). Lakehead University and Thunder Bay Regional Health Research Institute provided partial support and access to their facilities.References
- www.who.int
- Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nature Reviews Cancer 3,2003; 330-338.
- McIntyre D.J.O., et al. Can localised 19F magnetic resonance spectroscopy pharmacokinetics of 5FU in colorectal metastases predict clinical response? Cancer Chemother Pharmacol. 2011; 68:29-36.
- Arbuck, S.G. Overview of clinical trials using 5-fluorouracil and Leucovorin for the treatment of colorectal cancer.Cancer. 1989; 63:1036-1044
- Wolf W, Waluch V, Presant AC. Non – invasive 19F – NMRS of 5-fluorouracil in pharmacokinetics and pharmacodynamic studies. NMR Biomed. 1998; 11: 380-387.
- Lovis JA., et al. In vivo Chemical Shift Imaging of 5-Fluorouracil and it’s Metabolites. Proc. ISMRM. 2014; 3765.
- Otake Y., et al. In-vivo 19F Imaging of 5-Fluorouracil and its Metabolites in Rat by Two-Element Phased-Array Coil. Proc. ISMRM. 2011; 477.
- Presant, C. A., et al. Association of intratumoral pharmacokinetics of fluorouracil with clinical response. Lancet. 1994; 343(8907):1184-1187.
Figures