3998
Dietary modulation of metabolic enzyme activity assessed by dynamic in vivo 31P-MRS of hepatic fructose metabolism1Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Pfizer Worldwide Research & Development, Cambridge, MA, United States
Synopsis
In this study, 31P-MRS was applied to monitor hepatic fructose metabolism of rats in response to IV fructose challenge. Animals exposed to 7-days of sucrose-enriched diet experienced enhanced fructose clearance relative to animals on an isocaloric control diet. The finding of more efficient fructose metabolism in the sucrose fed group corresponded with elevated KHK and AldoB gene expression, two key enzymes responsible for fructose clearance. Treatment of animals on sucrose diet with a KHK inhibitor nearly completely blocked fructose metabolism and prevented increased expression of these enzymes, implying that metabolism of fructose elicits the enzyme induction rather than fructose itself.
Introduction
Fructose is a nearly ubiquitous component of the modern Western diet, and its consumption is argued to be a contributing factor to the rise in prevalence of obesity that afflicts roughly one-third the population of developed countries (1). Given the wide range of comorbidities associated with obesity and their cumulative economic and psychosocial implications, identifying viable options to reverse disease progression remains a significant healthcare need. One strategy could entail modulating the metabolic effects associated with dietary intake of fructose. Fructose metabolism is not subject to the same level of regulatory feedback inhibition as glucose metabolism; therefore fructose more readily enters the glycolytic pathway, facilitating de novo lipogenesis (DNL) and the accumulation of fat in storage depots such as the liver and adipose tissue (2). Metabolic enzyme expression is regulated through a network of factors that respond to both hormonal and nutrient status. In the case of fructose, metabolism is initiated by ketohexokinase (KHK) mediated phosphorylation of fructose to fructose-1-phosphate (F-1-P), which is further metabolized by aldolase B (AldoB), progressing through a series of intermediate metabolites prior to entering the glycolytic pathway. 31P-MRS has previously been applied to monitor the kinetics of fructose metabolism in the liver in response to IV fructose challenge by quantifying spectral change in the phosphomonoester peak (PME), which is attributed to change in F-1-P concentration (3). Here we report findings from a mechanistic study of the effects of chronic sucrose consumption on enzyme activity in rats as assessed by dynamic in vivo 31P-MRS of the liver.Methods
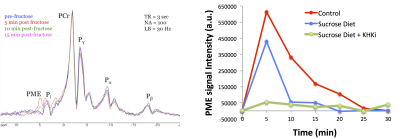
Adult Sprague-Dawley rats (male, 225-250g, ~8-weeks old) were maintained on either normal chow or an isocaloric sucrose diet consisting of 30% sucrose (D13032001, ResearchDiets) for a period of 7-days (N=6 per diet group). A separate group of rats (N=6) was treated with an inhibitor of KHK (30 mg/kg/day, PO) while on sucrose diet. Acquisition of 31P-spectra of rat liver was optimized on a Bruker Biospec 9.4T/20 to enable longitudinal tracking of fructose metabolism following IV administration of fructose bolus. A custom fabricated Tx/Rx 31P RF surface coil designed to rest under the abdomen of prone positioned rats and a standard pulse-acquire sequence with TR=3000 msec, SW=10 kHz, and NA=100 was used to dynamically acquire spectra with a temporal resolution of 5-minutes. In preparation for scanning, overnight fasted animals were anesthetized under isoflurane and a tail vein catheter was placed for in-bore administration of fructose solution (20% w/v, 500mg/kg). Baseline 31P-spectra were acquired, and post‑fructose metabolic response was followed through the collection of additional spectra over a period of 30-minutes. Difference spectra were generated by subtracting the average of the baseline spectra from the post-fructose spectra, and ΔPME response was quantified by calculating the area of the PME region (iNMR, MestreLab Research, S.L.). A separate study was conducted with identical treatment groups and duration to quantify alterations in gene expression. Following the 7-day treatment period, animals were fasted overnight, sacrificed, and hepatic RNA was isolated for gene expression analysis by qRT-PCR.Results
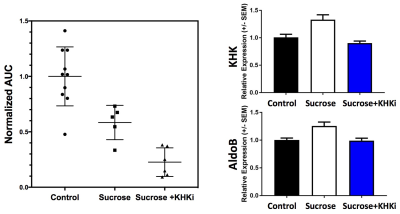
A stacked-plot time-series of spectra from an example dynamic run with a control diet animal is provided in Fig. 1A, along with representative plots of ΔPME response from animals of each study group in Fig. 1B. From the calculated ΔPME AUC values provided in Fig. 2A, it is noted that the chronic sucrose diet resulted in a statistically significant reduction in AUC relative to animals on normal chow (p<0.005), while the addition of a KHK inhibitor to the diet regimen resulted in a further reduction in ΔPME AUC. KHK and AldoB expression profiles from harvested livers of each treatment group are provided in Fig. 2B and demonstrate elevated levels in the sucrose diet group, and that inhibition of fructose metabolism prevented the induction of enzyme expression.Discussion
Data from this study suggests that 7-days of elevated sucrose ingestion promoted hepatic expression of KHK and AldoB, the two key enzymes responsible for fructose clearance. Induction of these enzymes facilitates the efficient metabolism of fructose, which is evident from the reduced ΔPME AUC observed in sucrose fed animals. In order to better discern if fructose itself or a metabolite of fructose promotes induction of these enzymes, a group of animals was treated with a KHK inhibitor while on the sucrose diet. Treatment with the KHK inhibitor nearly completely blocked fructose metabolism, as indicated by a ~75% reduction in DPME AUC relative to control diet animals. Additionally, the induction of KHK and AldoB was completely suppressed in the KHK inhibitor treated group, suggesting that fructose metabolism is required for increased enzyme expression.Acknowledgements
No acknowledgement found.References
1. NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016: 387:1377-1396.
2. Sun SZ, et al. Fructose metabolism in humans – what isotopic tracer studies tell us. Nutr Metab 2012; 9:89.
3. Boesiger P, et al. Changes of liver metabolite concentrations in adults with disorders of fructose metabolism after intravenous fructose by 31P magnetic resonance spectroscopy. Pediatr. Res. 1994; 36:436-440.
Figures