3992
In vivo Phosphorus Metabolite Imaging on a 3T MRI Scanner in a Clinically Feasible Scan Time1Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
We developed an MRI system (coil, sequence, reconstruction) capable of performing imaging of phosphorus metabolites in a clinically-feasible scan time on a conventional 3T MRI scanner. Each component of the system is explained, and results of a phantom scan and scan of a human thigh are presented.
INTRODUCTION
While conventional MRI is good for diagnosing and monitoring many diseases, there is a large unmet clinical need for imaging methods that can detect relevant metabolic abnormalities in disease not visible using conventional MRI. Adenosine triphosphate (ATP) and phosphocreatine (PCr) are intracellular phosphorus-containing compounds involved in cellular energy metabolism that are abnormal in many diseases including cancer and stroke1,2,3. However, due to their relatively low intracellular concentrations and poor MR sensitivity of phosphorus (6.7% that of protons), detecting these metabolites with MRI at 3T has been difficult at best with MR spectroscopy and nearly impossible with MR imaging. We hypothesize that clinically useful phosphorus MRI (PMRI) can be performed on a standard 3T MRI system in a clinically reasonable scan time.MATERIALS/METHODS
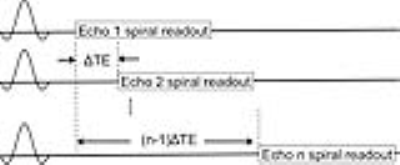
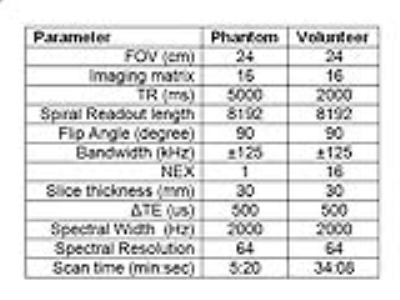
Hardware: A dual tuned proton/phosphorus Quadrature Transmit Array Receive (QTAR) head coil (Clinical MR Solutions, Brookfield, WI) was used. This coil system has 8 phosphorus receive channels and a single proton quadrature receive channel (Figure 1). The coil was interfaced with a 3T MRI scanner (GE Discovery MR750, GE Healthcare, Waukesha, WI). A two-peak phosphorus phantom was constructed by mixing 50 mM phenylphosphonic acid with 100 mM phosphoric acid in a small vial. Sequence: For PMRI experiments, an axial 2D spiral chemical shift imaging (CSI) sequence was used4. Following each RF excitation, a single-shot spiral readout was used to capture the 2D k-space. The echo time was sequentially shifted by ΔTE between excitations to achieve spectral encoding (Figure 2). The spectral resolution was determined by the number of time-shifted echoes and ΔTE. An additional free induction decay acquisition without readout gradients was acquired to obtain knowledge about the spectrum for reconstruction. The two peak phantom and, with IRB approval, the thigh of a healthy volunteer were scanned using the spiral CSI sequence. The imaging parameters for both phantom and volunteer experiments are listed in the table (Figure 3). Reconstruction/Denoising: Image reconstruction was performed using modified existing MNS software, which nominally performs gridding IDEAL species separation, and coil-combine sequentially4. Our developed reconstruction model directly and simultaneously estimates a multi-channel, multi-species data set from k-space data via locally low-rank5 regularized least-square regression with embedded non-uniform fast Fourier transform (NUFFT)-based gridding6. This strategy generalizes earlier compressed sensing MNS reconstructions7 for calibrationless parallel imaging, and prospectively utilizes optimal coil combine concepts8.RESULTS
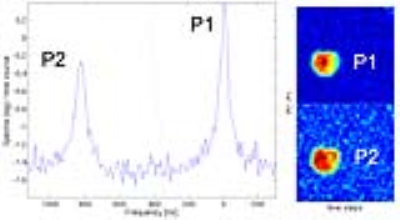
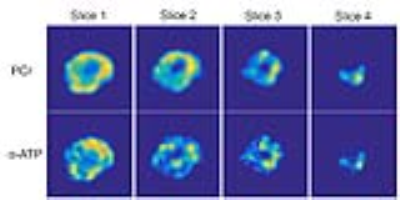
Initial phosphorus MRS (PMRS) of the two-peak phantom (Figure 4, left) showed an 824 Hz (16 ppm) separation between peaks as expected, with the “half height” PPA peak (P2) on the left and the “full height” phosphoric acid peak (P1) on the right. PMRI of the phantom (Figure 4, right) showed good SNR of the two compounds. PMRI images obtained from the volunteer experiment are shown in Figure 5. These consisted of 4 axial slices reconstructed from the spiral CSI sequence, focusing on high energy metabolites including PCr (central frequency) and α-ATP (-7.8 ppm). Skeletal muscle has higher levels of PCr, hence the relatively increased signal intensity of the PCr images relative to the α-ATP images.
DISCUSSION/CONCLUSION
We have successfully created a PMRI imaging system consisting of an 8-channel phosphorus head coil, spiral CSI pulse sequence and new reconstruction/denoising algorithm. Such PMRI images are indeed possible within a clinically feasible scan time, with potential further scan time reductions possible through sequence optimization as well as incorporation of a proton decoupler. This system opens up new possibilities for metabolic imaging, and may someday become a clinically essential imaging tool for the diagnosis and treatment of diseases with metabolic rather than structural abnormalities.Acknowledgements
The authors acknowledge the significant coil design contributions of Ralph Hashoian from Clinical MR Solutions.References
1Martinez-Outschoorn U, Peiris-Pages M, Pestell RG, et al. Cancer metabolism: a therapeutic perspective. Nature Reviews Clinical Oncology 14:11-31, 2017; 2Zhou Y, Tozzi F, Chen J, et al. Intracellular ATP Levels are a Pivotal Determinant of Chemoresistance in Colon Cancer Cells. Cancer research. 2012;72(1):304-314.; 3Bolas NM, Rajagopalan B, Mitsumori F, Radda GK. Metabolic changes during experimental cerebral ischemia in hyperglycemic rats, observed by 31P and 1H magnetic resonance spectroscopy. Stroke. 1988 May;19(5):608–614; 4Wiesinger, F., Weidl, E., Menzel, M. I., et al. (2012), IDEAL spiral CSI for dynamic metabolic MR imaging of hyperpolarized [1-13C]pyruvate. Magn. Reson. Med., 68: 8–16.; 5Trzasko, Joshua D., and Armando Manduca. "Calibrationless parallel MRI using CLEAR." Signals, Systems and Computers (ASILOMAR), 2011 Conference Record of the Forty Fifth Asilomar Conference on. IEEE, 2011.; 6 Fessler, Jeffrey A., and Bradley P. Sutton. "Nonuniform fast Fourier transforms using min-max interpolation." IEEE Transactions on Signal Processing 51.2 (2003): 560-574.; 7Wiens, Curtis N., et al. "Chemical shift encoded imaging of hyperpolarized 13C pyruvate." Magnetic resonance in medicine 74.6 (2015): 1682-1689.; 8Rodgers, Christopher T., and Matthew D. Robson. "Coil combination for receive array spectroscopy: Are data‐driven methods superior to methods using computed field maps?." Magnetic resonance in medicine 75.2 (2016): 473-487.
Figures