3991
First experiences with a phosphorus 30 channel receiver head array at 7T1CUBRIC, University of Cardiff, Cardiff, United Kingdom, 2Radiology, UMC Utrecht, Utrecht, Netherlands, 3MR Coils, Zaltbommel, Netherlands
Synopsis
Multi-receiver arrays offer the high sensitivity of surface coils and the extended field of view of volume coils, as long as the individual receivers can be combined correctly. In 31P MRS, the low intrinsic SNR makes determining weighting factors challenging and data quality can suffer. We report on our experiences of working with a custom-built 30 channel head coil, dual-tuned for 1H and 31P at 7T, comparing its performance against a traditional birdcage, in phantom and in vivo. Our results are encouraging, with equal SNR in the centre of the brain and significantly enhanced signal in the periphery.
Introduction
Magnetic Resonance Spectroscopy with 31Phosphorus is an important non-invasive tool for studying cellular energy metabolism, cell-membrane turnover and intracellular pH. The primary challenge in 31P MRS is the low signal-to-noise-ratio (SNR) arising from the lower gyromagnetic ratio and low concentration of the metabolites of interest. 7T offers a substantial increase in SNR and increased spectral dispersion relative to lower field strengths.
The majority of 31P MRS studies at 7T use either surface coils, which provide excellent sensitivity in a restricted field of view, or birdcage coils, which offer a broad field of view but reduced sensitivity. By making use of a multi-channel receiver array, the improved sensitivity of surface coils can be extended over a wider field of view, provided the data from individual channels can be combined correctly[1]. In this work we describe our initial results working with the first (to our knowledge) 31P 7T head coil with a 30 channel receiver array.
Methods
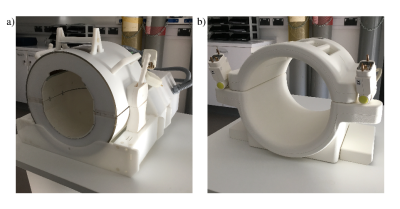
The 30 channel coil setup was custom built by MR Coils (Utrecht, Netherlands). Each receive element was double-tuned to 31P and 1H, incorporating active detuning, and per nucleus driven to narrow band preamplifiers. For transmit a bilayer detunable birdcage coil was used, each layer tuned to one nucleus but with bandstop filters to prevent crosstalk. For this study, we compared this novel coil against a standard single channel birdcage coil provided by Quality Electrodynamics (Ohio, USA). Both coils are shown in Figure 1. All described scans were performed identically using both coils on a 7T Siemens Magnetom scanner (Erlangen, Germany).
Phantom: a 2l spherical flask was filled with a 16mM solution of phosphate buffer (pH=7.2) and scanned using a non-selective 3D CSI sequence with: FOV 200x200x200, matrix 16x16x16, TR/TE 1000/2.3, flip angle 30, spherical k-space sampling, acquisition time 23 minutes.
In vivo: three healthy volunteers were scanned. In two a transverse slice was acquired, while in the third the slice was positioned more coronally through the occipital and posterior cingulate cortices. The acquisition was a slice-selective 2D CSI sequence with: FOV 200x200x40, matrix 12x12, TR/TE 1000/2.3, flip angle 45, weighted k-space sampling with 60 averages, acquisition time 24 minutes.
B1 power was calibrated manually by determining the power required to give maximum signal from the whole slice following a 0.5ms pulse. In post-processing, for the array coil channel combination was done voxel by voxel using the WSVD+Apod method[2], then datasets from both coils were automatically phased, scaled to a fixed level of noise and the metabolites fitted using a singlet fitting method. All processing was done using Suspect[3].
Results
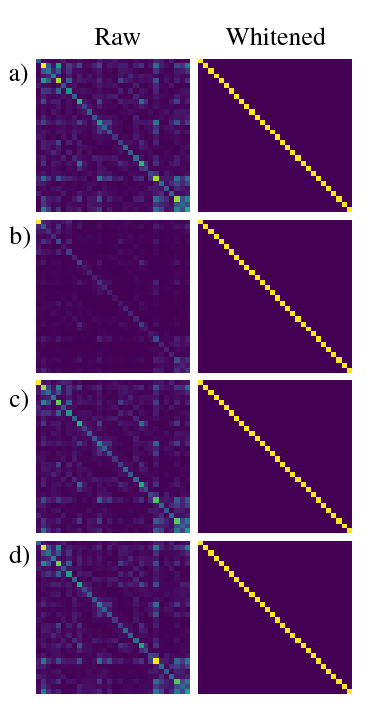
Figure 2 shows channel noise correlation matrices for each scan. The raw data shows some correlation between channels and variable coil loading, however post-whitening all channels are uncorrelated and equally loaded.
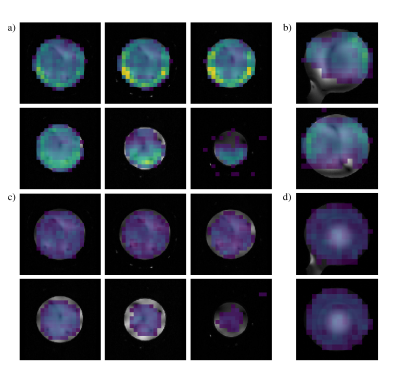
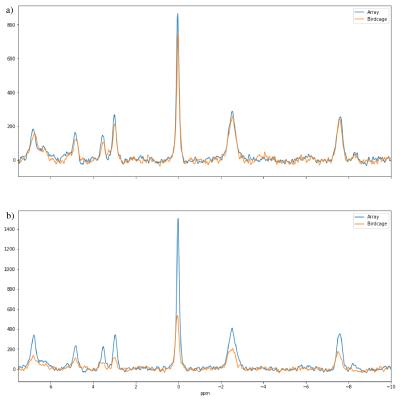
Phosphocreatine SNR maps for the phantom acquisition are shown in Figure 3. In the centre of the volume SNR was 1.2x higher for the array coil, increasing to more than 8x towards the occipital region. In the superior half of the phantom SNR was universally greater, but coverage is worse lower in the coil.
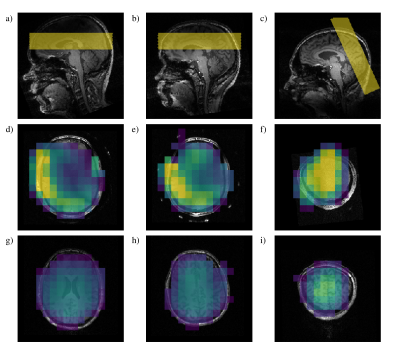
Results for the in vivo data (Figures 4&5) show a similar pattern: for the occipital slice we obtained universally higher SNR with the array coil, from around 1.5x in the centre of the slice to more than 4x in the periphery. In the transverse slice the SNR of the central voxels was around 80% relative to the birdcage, but with SNR increases exceeding 6x at the edges of the brain.
Discussion
Correct channel weightings are essential to obtain good spectra from an array coil, however performance of current algorithms is predicated on sufficient SNR from each channel. In our phantom scans SNR was high enough to ensure correct channel combination, however for our volunteer scans, centre of brain SNR was insufficient and overall quality was worse. More optimal combination strategies should improve the performance in vivo.
SNR in the centre of the brain was slightly lower with the array than the birdcage, however the comparatively high SNR there means this minor decrease is less important: by comparison the increase in SNR in the periphery is more significant, as it increases the spatial region with quantifiable levels of signal.
Conclusion
The use of a 30 channel receiver array for measuring 31P in the brain at 7T gives substantial improvements in SNR at the periphery of the brain while maintaining performance in the centre, relative to a conventional birdcage, however improved methods for channel combination will be required to achieve higher resolutions and/or shorter scan times.Acknowledgements
We gratefully acknowledge the assistance of Alison Paul and Craig James in the preparation of phantoms.References
[1] van de Bank, B. L., Orzada, S., Smits, F., Lagemaat, M. W., Rodgers, C. T., Bitz, A. K., & Scheenen, T. W. J. (2015). Optimized 31P MRS in the human brain at 7 T with a dedicated RF coil setup. NMR in Biomedicine, 28(11), 1570–1578
[2] Rodgers, C. T., & Robson, M. D. (2015). Coil combination for receive array spectroscopy: Are data-driven methods superior to methods using computed field maps? Magnetic Resonance in Medicine, 75(2), 473–487
[3] Rowland B. (2017) OpenMRSLab: An open-source software repository for Magnetic Resonance Spectroscopy data analysis tools. Proceedings of the 34th Annual Meeting of ESMRMB
Figures