3990
Unambiguous detection of cardiac Pi using long TM 31P STEAM1OCMR, RDM Cardiov. Medicine, University of Oxford, Oxford, United Kingdom, 2CMPBME, MR Physics, MRCE, Medical University of Vienna, Vienna, Austria, 3Institute of Measurement Sciences, Department of Imaging Methods, Slovak Academy of Science, Bratislava, Slovakia, 4Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom
Synopsis
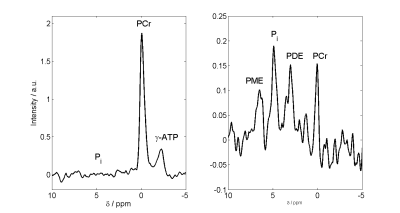
Inorganic Phosphate is a resonance that holds important information on the metabolic state of tissues. From its resonance frequency, intracellular pH can be derived. The ratio of Pi to PCr or ATP are also important markers. Unlike in other tissues, myocardial Pi is frequently hidden underneath blood 2,3-DPG signals. Using 7 T STEAM's TM delay to be one cardiac cycle, blood-pool originating signals are gone and the Pi resonance is clearly visible. In 3 subjects, Pi signal was detected and quantified. The signal was around 4.89±0.02ppm, corresponding to a pH of 7.08±0.02. This is a breakthrough for the investigation of cardiac metabolism.
Introduction
Investigating cardiac metabolism and its pathological alterations are essential in understanding various diseases. Cardiac 31P MRS is one of very few techniques capable of measuring cardiac metabolism in-situ and non-invasively. So far, the inorganic phosphate signal (Pi), commonly found in 31P MRS data has escaped unequivocal detection. The aim of this project is to be able to reliably quantify Pi and hence pH in human subjects at 7 T using 31P MRS.
In protocols with short acquisition delay or TE contributions from blood-pool 2-3 diphospho-glycerate (DPG) signals around 5.5 ppm give rise to overlapping resonances due to imperfect localisation and limited spectral resolution [1]. The STEAM sequence offers two interesting properties: First, 90 deg RF pulses are comparatively easy to achieve, second, the magnetisation during the TM time is stored along the longitudinal axis where T1 decay occurs. Pi has a long T1 of about 5.1 s [2]. Long TM short TE STEAM should give rise to signal from myocardium while suppressing fast moving blood.
Methods
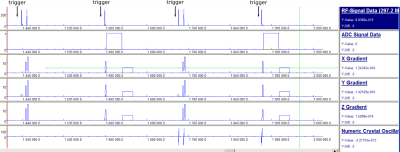
A 7 T (Magnetom, Siemens, Germany) STEAM sequence (Figure 1) was adapted to ECG triggering twice, before the first and the third pulse, resulting in an effective TM of one heartbeat. Thereby, a significant fraction of the blood pool has left the voxel before the echo is acquired. Furthermore, the spoiler gradients during the TE intervals act as motion-sensitising gradients, probably contributing significantly to blood suppression [3]. Unfortunately, the weak B1+ field (16 channel array, RAPID, Germany) requires comparatively long pulses (4.5 ms truncated sinc shapes) that produce significant chemical shift displacement artefacts. This was circumvented using interleaved acquisitions with shifted excitation: One at 0 Hz (PCr) and one at 570 Hz (Pi) every TR (Figure 1). Effective repetition times for each metabolite were six heart-beats.
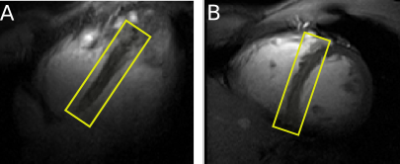
Healthy subjects were scanned lying supine. Localiser images in the main cardiac orientations were acquired before spectroscopy acquisition. Voxels were placed to cover the septum; dimensions varied between subjects (148±86 ml) (Figure 2). WSVD [4] coil combination was used based on the PCr signal in both scans. Matlab AMARES [5] was used to fit peaks, first the PCr-only spectrum and then Pi, whose line widths was constrained to that of PCr. The phase of PCr was used as an initial value for the Pi phase. This helped the fit converge to reasonable values judged by visual inspection.
Results and Conclusions
Cardiac 31P single voxel spectroscopy has not been attempted at 7 T before, we think. Unlike in UTE-CSI data (Figure 3), the 2,3 DPG signal is gone and Pi nicely observable (Figure 4). Using this approach, PCr and in particular Pi signals were measured in 3 subjects (SNR Pi > 4, SNR PCr > 50), so far. Cardiac intracellular pH values were 7.078 ± 0.017. The Pi signal is 0.09 ± 0.02 times weaker than PCr. These values are very similar to those reported in patients with hypertrophic cardiomyopathy (HCM) by high resolution CSI [6].
Unlike previous reports, this technique resulted in spectra with Pi clearly visible and without a predominant 2,3-DPG signal in the 5 ppm spectral area in all subjects after a few minutes' scan time even in healthy subjects with low Pi concentrations and normal left myocardial wall thickness (unlike the HCM patients mentioned above). The chemical shift displacement is really quite strong so even ATP in the PCr scan is significantly affected. The small PCr peak in Figure 4 is a residual originating from some completely different location.
The ability to non-invasively quantify intracellular cardiac Pi in a matter of minutes will be a significant boost to the utility of cardiac 31P MRS and a new tool for cardiology research.
Acknowledgements
This work was supported by the Austrian Science Fund (FWF) project J 4043 as well as by the Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (098436/Z/12/Z),References
[1] CT Rodgers et al. Magn Reson Med; 2014; doi: 10.1002/mrm.24922
[2] L Valkovic et al. “Quantification of human cardiac Pi”; submitted to ISMRM 2018
[3] TE Reese et al. Magn Reson Med 1995; doi: 10.1002/mrm.1910340603
[4] CT Rodgers et al. Magn Reson Med 2010; doi: 10.1002/mrm.22230
[5] LAB Purvis et al. PloS One 2017; doi: 10.1371/journal.pone.0185356
[6] WT Clarke “Human cardiac magnetic resonance spectroscopy“; 2016; http://solo.bodleian.ox.ac.uk/OXVU1:LSCOP_OX:oxfaleph020840395
Figures
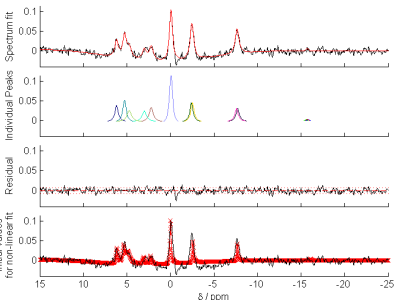
- Typical cardiac 31P spectrum (and OXSA fitting results) from a 3D CSI pulse-acquire scan from a healthy volunteer at 7T. Pi is barely visible as a shoulder to the 2,3-DPG peak at around 5 ppm.