3985
Electrophysiological stimulation of excised rat muscle elicits a measurable change in tissue sodium concentration using 23Na-MRI1Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 2Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom, 3Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 4GE Healthcare, Munich, Germany, 5Department of Physiology, Development and Neuroscience, University of Cambridge, Cambridge, United Kingdom
Synopsis
Changes in the tissue sodium gradient play an important role in cell signalling such as at the neuromuscular junction and as part of neuronal action potentials. 23Na-MRI has the ability to measure the macroscopic sodium distribution. In this study we investigated the changes in tissue sodium in an electrically stimulated and freshly excised rat leg muscle.
Introduction
Changes in the tissue sodium gradient play an important role in cell signalling such as at the neuromuscular junction and as part of neuronal action potentials. 23Na-MRI has the ability to measure the macroscopic sodium distribution. In this study we investigated the changes in tissue sodium in an electrically stimulated and freshly excised rat leg muscle.Methods
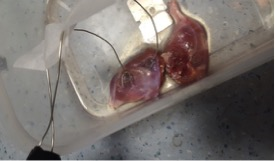
The rat (Lister, age: 14 weeks) was sacrificed according to local ethical rules. Rat hind leg muscles were obtained within 1 hour of imaging. Muscles were placed in a container filled to 1 mm depth with buffer solution1 (fig.1). The container was placed on top of a custom 23Na T/R coil of 15 cm diameter in a clinical 3T MRI (GE MR750, GE Healthcare, Waukesha, WI). A 3D-Cones sequence2 was used for 23Na-imaging (resolution = 3.8x3.8x3.8 mm3, FOV = 20 cm, TE/TR = 0.5/50 ms, flip angle = 90°, readout length = 30 ms, 197 readouts, duration of one scan = 10 seconds). A waveform generator was placed outside of the room, and the cable connected to an MRI pass panel to stimulate the muscle with prongs attached to the muscle for field excitation (20 ms pulses, 1 second between pulses). A trap circuit at 33 MHz (>30 dB attenuation) was placed on the cables to remove noise at the imaging frequency for sodium. Images were corrected for B1 sensitivity using the double angle method (FA = 30° and 60°)3. 20 and 85 mM NaCl in 4% agar phantoms were placed in the FOV for quantification4. Imaging was performed in 1 minute blocks (6 volumes per block), 4 blocks in total (2 blocks of stimulation interleaved with 2 rest blocks). SPM12 (UCL, London, UK) was used to find voxels undergoing significant sodium signal changes in the FOV. No response modelling function (such as hemodynamic response) was used due to the instantaneous nature of the sodium flux and low temporal resolution (10 s).Results
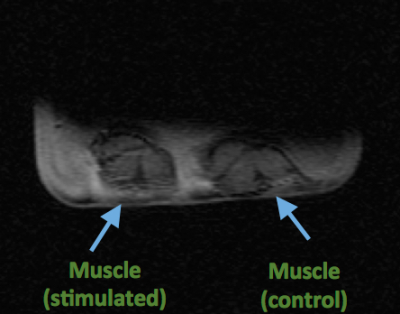
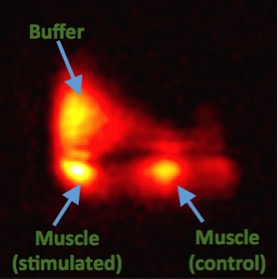
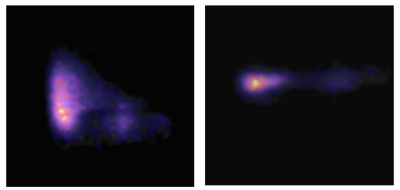
Figure 2 shows a 1H image of the set up. The short 23Na-MRI scan (10 s per volume) yielded sufficient SNR (≈ 22 a.u. ; fig. 3). The average total sodium concentration in the muscle tissue at the beginning and end of the experiment was 37 mM. A change in sodium concentration between +1 to + 4 mM was detected during stimulation (p < 0.001, FWE corrected), fig.4. Muscle tetany during stimulation was confirmed visually. No signal changes were detected in the control muscle which was not stimulated.Discussion
The results demonstrate for the first time that rapid sodium signal changes can be detected within excited skeletal muscle using 23Na-MRI. In this experiment an alteration in tissue sodium was detected which corresponds to the sodium flux during activation of the neuromuscular junction. As the muscle was imaged ex vivo, contribution of blood flow (also a carrier of sodium ions) should not have an effect.Conclusion
Here we have demonstrated that non-invasive 23Na-MRI has the sensitivity and temporal resolution to be used to study functional changes in sodium concentration in muscle tissue during stimulation. This method could be applied in the future to investigate neuromuscular diseases where alterations in sodium transport are known to occur e.g. myopathies. This observation in muscle provides further evidence that 23Na-MRI can be used as a functional measure of action potential activity within the central nervous system, which would complement traditional proton functional MRI measurements within the brain5.Acknowledgements
This work was supported by CRUK [C8742/A18097]. This is a contribution from the Cancer Imaging Centre in Cambridge & Manchester, which is funded by the EPSRC and Cancer Research UK. We would like to express our gratitude to the Experimental Cancer Medicine Centres (ECMC) for continued support. JK receives funding support from GlaxoSmithKline.References
1. Pedersen TH, de Paoli F, Nielsen OB. Increased Excitability of Acidified Skeletal Muscle: Role of Chloride Conductance. Journ. Gen. Phys. 2005; 125(2):237–46.
2. Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a Practical 3D Cones Trajectory. MRM 2006; 55:575-582.
3. Sled JG, Pike GB. Correction for B(1) and B(0) variations in quantitative T(2) measurements using MRI. MRM 2000; 43:589-593.
4. Christensen JD, Barrere BJ, Boada FE, Vevea JM, Thulborn KR. Quantitative Tissue Sodium Concentration Mapping of Normal Rat Brain. MRM 1996; 36:83-89.
5. Riemer F, Solanky BS, Golay X, D’Angelo EU, Wheeler-Kingshott CAM. Sodium fMRI detects grey and white matter activations: neuronal firing or blood volume change? In Proc. Int. Soc. Magn. Reson. Med. 2015, 23:3924.
Figures