3972
Simultaneous editing of GABA and glutathione at 7T1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Biomedical Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
HERMES has been shown to simultaneously edit GABA and GSH at 3T. Spectral editing at 7T provides better editing pulse selectivity, e.g. quantifying GABA with less contamination from macromolecules. In this abstract, simulations, phantom and in vivo experiments were performed at 7T using the
Purpose
γ-aminobutyric acid (GABA) and glutathione (GSH) are implicated in a range of neurological diseases1,2, and are the most commonly edited metabolites. Despite the benefit of higher intrinsic signal-to-noise ratio (SNR), ultra-high-field magnetic resonance spectroscopy (MRS) also suffers from technical challenges such as increased chemical shift displacement error (CSDE) and transmit-field (B1) inhomogeneity3, adversely affecting voxel localization and editing efficiency. Recent studies have demonstrated spatially accurate and efficient editing at 7T, combining Mescher-Garwood (MEGA) editing and semi-localization by adiabatic selective refocusing (sLASER)4. MEGA-sLASER targets a single metabolite per experiment and is usually applied to lower-concentration compounds, such as GABA and GSH, for which long acquisition times are necessary, limiting the number of brain regions and metabolites that can be studied within the constraints of a typical MR examination. The purpose of this study, through simulation, phantom and in vivo experiments, was to accelerate spectral editing at 7T by simultaneous editing of GABA and GSH, using Hadamard encoding and reconstruction of MEGA-edited spectroscopy (HERMES)5.Methods
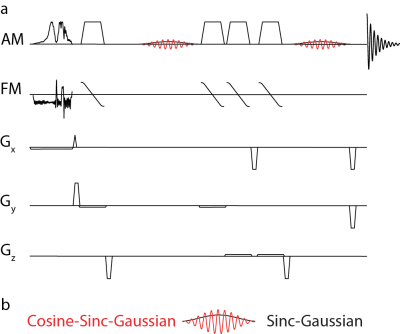
The HERMES-sLASER sequence is shown in Figure 1. HERMES of GABA and GSH consists of four sub-experiments: (Experiment A: 1.9 ppm for ONGABA, 4.56 ppm for ONGSH), GABA-only (Experiment B: ONGABA, OFFGSH), GSH-only (Experiment C: OFFGABA, ONGSH) or neither (Experiment D: OFFGABA, OFFGSH). The GABA-edited difference spectrum is derived from A+B-C-D, while the GSH-edited difference spectrum is derived from A-B+C-D. The duration and bandwidth (or sweep-width) of the pulses are given in the caption for Figure 1.
Simulation: Non-localized simulations were performed assuming ideal slice-selective excitation and shaped refocusing and editing pulses using spin parameters from Tkac6 for GSH and Near et al.7 for GABA. The simulation parameters were as follows: TE = 80 ms; peak B1 = 15 μT; 8192 points; spectral width (SW) = 5 kHz; simulated linewidth = 2 Hz; additional line broadening using a 3-Hz exponential filter.
Phantom: A phantom containing phosphate-buffered saline with 10 mM GABA and 10 mM GSH was prepared. A HERMES-sLASER experiment was performed using the following acquisition parameters: TR = 3000 ms; TE = 80 ms; peak B1 = 15 μT; SW = 5 kHz; 3 x 3 x 3 cm3 voxel; 16 averages per sub-experiment; and VAPOR water suppression.
In vivo: 10 healthy adults were scanned with the approval of the local institutional review board after giving informed consent to participate. HERMES, GABA-MEGA- and GSH-MEGA-edited data were acquired from a single 27-mL voxel in the medial parietal lobe with the same acquisition parameters as for the phantom experiments, except 224 total averages (56 per sub-experiment for HERMES, 112 ON and 112 OFF for MEGA-sLASER) were acquired. The scan duration of each acquisition was ~11 min.
Post-processing: Data were frequency-and-phase aligned in the time domain8. Subsequent to alignment, data were filtered with a 5-Hz exponential function and the GABA and GSH concentrations were quantified relative to the unsuppressed water signal from the same volume.
Results
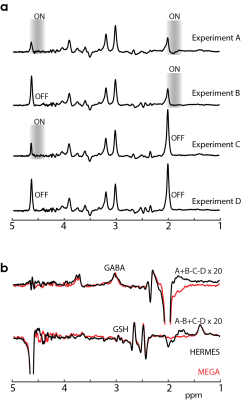
Simulations of HERMES indicate that it is possible to acquire GABA- and GSH-edited spectra at the same time, with negligible levels of crosstalk (Figure 2). Phantom experiments also demonstrate well-edited GABA and GSH difference spectra, in agreement with corresponding simulations. In vivo experiments using HERMES-editing scheme reveal well-edited GABA and GSH signals, and in excellent agreement with sequentially acquired MEGA-sLASER data with twice the total duration (Figure 3). The average concentrations from HERMES edited-spectra (GABA/GSH: 1.051 ± 0.254/0.300 ± 0.091 i.u) are not statistically different (p = 0.985/0.940) from the MEGA-sLASER edited-spectra (1.053 ± 0.248/0.302 ± 0.093 i.u).Discussion
HERMES along with sLASER localization allows simultaneous measurements and excellent separation of GABA and GSH at 7T with half the acquisition time compared to sequential MEGA-editing. Increased dispersion of the chemical shift axis at 7T allows better editing pulse selectivity for a given finite pulse duration. Thus, HERMES editing at 7T results in a GABA-edited signal with a smaller signal contribution from macromolecules. sLASER gives superior localization at all field strengths, compared to PRESS. 7T editing does suffer from shorter in vivo T2s, and therefore in this study, GABA and GSH were edited at the shortest possible TE (80 ms). Further enhancements can be achieved by incorporating adiabatic pulses with increased bandwidth to further reduce CSDE and improve editing efficiency9.Conclusion
HERMES with sLASER localization at 7T allows simultaneous editing of the two most commonly investigated metabolites, GABA and GSH, in half the total scan time, and without any loss in spectral quality compared to MEGA-sLASER editing. Compared to prior work, editing at 7T is more selective than at 3T, and sLASER provides better localization than PRESS.Acknowledgements
This work was supported by NIH grants R01 EB023963 and P41EB015909.References
1. Levy LM, Degnan AJ. GABA-based evaluation of neurologic conditions: MR spectroscopy. AJNR Am J Neuroradiol 2013;34:259-265.
2. Shungu DC. N-acetylcysteine for the treatment of glutathione deficiency and oxidative stress in schizophrenia. Biol Psychiatry 2012;71:937-938.
3. Scheenen TW, Klomp DW, Wijnen JP, et al. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med 2008;59:1-6.
4. Prinsen H, de Graaf RA, Mason GF, et al. Reproducibility measurement of glutathione, GABA, and glutamate: Towards in vivo neurochemical profiling of multiple sclerosis with MR spectroscopy at 7T. J Magn Reson Imaging 2017;45:187-198.
5. Chan KL, Puts NA, Schar M, et al. HERMES: Hadamard encoding and reconstruction of MEGA-edited spectroscopy. Magn Reson Med 2016;76:11-19.
6. Tkac I. Refinement of simulated basis set for LCModel analysis. In Proceedings of the 16th Annual Meeting of ISMRM, Toronto, Canada, 2008. p 1624.
7. Near J, Evans CJ, Puts NA, et al. J-difference editing of gamma-aminobutyric acid (GABA): simulated and experimental multiplet patterns. Magn Reson Med 2013;70:1183-1191.
8. Edden RA, Puts NA, Harris AD, et al. Gannet: A batch‐processing tool for the quantitative analysis of gamma‐aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging 2014;40:1445-1452.
9. Arteaga de Castro CS, Boer VO, Andreychenko A, et al. Improved efficiency on editing MRS of lactate and gamma-aminobutyric acid by inclusion of frequency offset corrected inversion pulses at high fields. NMR Biomed 2013;26:1213-1219.
Figures