3965
Impact of sub-echo timings of PRESS on quantitative glutamate/glutamine level using LCModel at 7T1Dept. of Computer Science and Engineering, National Sun Yat-sen University, Kaohsiung, Taiwan, 2Medical Physics,Department of Radiology, University Medical Center Freiburg, Freiburg, Germany
Synopsis
In this study, we investigated the signal variation of Glu and Gln along sub-echo timings (TE1, TE2) in PRESS acquisitions on a preclinical 7T system. Our results show that the choice of sub-echo timings (TE1, TE2) for PRESS may alter the quantitative outcome remarkably for strongly coupled metabolites, e.g. Glu, Gln. The basis-sets used for LCModel analysis has to be carefully simulated and take the sub-echo timings into account since J-evolution of strongly coupled resonances may vary with TE1/TE2 at high fields. Quantitative comparison on these metabolites with mismatched sub-echo timings (TE1, TE2) can result in invalid conclusion.
Introduction
Magnetic resonance spectroscopy (MRS) at high fields benefits from signal gain and enhanced spectral resolution. Application on clinical and preclinical studies using high-field MRS to detect metabolites with complex coupling profiles has been increasing recently1, 2. Optimization of TE timings of point-resolved spectroscopy (PRESS) for specific metabolites, e.g. glutamate (Glu), glutamine (Gln), GABA and 2HG has been reported1, 3. However, impact on Glu and Gln quantitation over various sub-echo timings at constant TE has not been mentioned. In this study, we investigated the signal variation of Glu and Gln along sub-echo timings (TE1, TE2) in PRESS acquisitions on a preclinical 7T system. With appropriate choice of TE, we demonstrated the different J-evolution of Glu, Gln at 2.0~2.5 ppm by simulation, phantom and in vivo experiments. The impact on quantitative analysis using LCModel was addressed.Methods
An open source IDE tool “VeSPA” (Versatile Simulation, Pluses and Analysis for Magnetic Resonance Spectroscopy)4 was used for spectral simulations, TE-space evaluation, and basis-sets generation for LCModel fitting. 2 Phantoms containing Glu and Gln individually were prepared and scanned to avoid interaction between Glu and Gln. DSS was also included in each phantom to ensure the Glu/Gln to be 3:1 in spectral combination. Phantom and in vivo (mouse) experiments were performed on a preclinical 7T MR system (Bruker BioSpec 70/20 USR) with a quadrature volume coil (inner diameter = 4 cm). Standard PRESS was applied with scan parameters: TR = 3000 msec, NEX = 256, voxel size = 2.5x2.5x2.5 mm3, bandwidth = 3300 Hz. Water suppression using VAPOR with RF bandwidth = 200 Hz was performed before PRESS. The TE value of 32 msec was designed by TE-space evaluation, along with two different TE1/TE2 settings in order to investigate the potential quantitative deviation of glutamine and glutamate. Acquired spectra were analyzed by LCModel without eddy current correction and water scaling. Therefore, quantitative concentrations were only comparable by ratios (Glu/Gln).Results
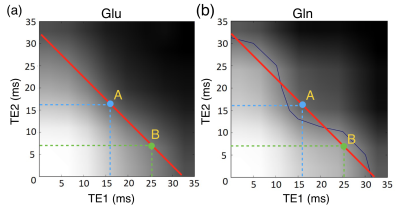
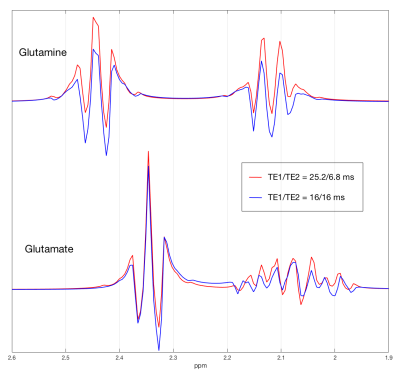
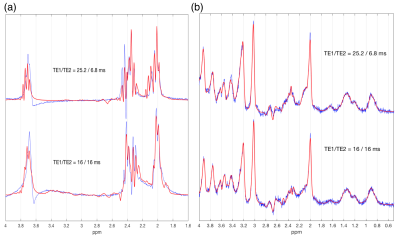
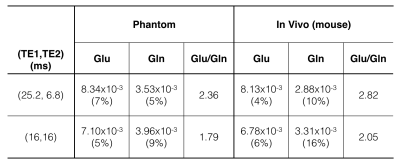
VeSPA simulation was performed to investigate TE space of TE1/TE2 ranging from 0 to 35 msec as shown in Fig. 1. The contour plots illustrated the signal variation along TEs between 1.8 to 2.8 ppm corresponding to PQ and MN resonances of Glu and Gln. TE=32 msec was chosen (red line in Fig. 1) because of the maximized signal variation caused by sub-echo timings. It was validated by spectra simulation using sub-echo timings (TE1, TE2) = (25.2, 6.8) and (16, 16) for Glu and Gln respectively (Fig.2). An evident J-evolution difference between two spectra of TE1/TE2 settings was observed on Gln while on Glu only minor change was found. In-vivo spectra were analyzed by LCModel with corresponding basis-sets (Fig.3b) without eddy current correction and water scaling. Phantom spectra were also included and processed with the same procedure for comparison (Fig.3a). Table 1 summarized quantitative results of phantom and in-vivo spectra. Concentrations of Glu and Gln were presented with arbitrary unit. The ratios of Glu/Gln were higher for PRESS (TE1, TE2) = (25.2, 6.8) with lower fitting errors (SD%) suggesting that LCModel performed better fitting at (TE1, TE2) = (25.2, 6.8) than at (TE1, TE2) = (16, 16) with constant TE = 32 msec. Additionally, Glu/Gln ratio (=2.82) of in-vivo spectrum at (TE1, TE2) = (25.2, 6.8) appears in coincidence with the values published in the literature5, whilst at (TE1, TE2) = (16, 16), the ratio was underestimated probably due to the poor fitting of Gln (SD = 16%) .Discussion and Conclusion
In this study, we investigated the impact on J-evolution of Glu and Gln arising from sub-echo timings of PRESS on a 7T MR system. Our results show that the choice of sub-echo timings (TE1, TE2) for PRESS may alter the quantitative outcome remarkably, especially for strongly coupled metabolites, e.g. Glu, Gln. Moreover, the basis-sets used for LCModel analysis has to be carefully simulated and take the sub-echo timings into account since J-evolution of strongly coupled resonances may vary along with TE1/TE2 at high fields3 even if the TE remains constant. Quantitative comparison on these metabolites with mismatched sub-echo timings (TE1, TE2) can result in invalid conclusion.Acknowledgements
No acknowledgement found.References
1. Ganji SK, An Z, Tiwari V, McNeil S, Pinho MC, Pan E, Mickey BE, Maher EA, Choi C. In vivo detection of 2-hydroxyglutarate in brain tumors by optimized point-resolved spectroscopy (PRESS) at 7T. Magnetic Resonance in Medicine, 2017;77(3):936-944.
2. Crescenzi R, DeBrosse C, Nanga RP, Reddy S, Haris M, Hariharan H, Iba M, Lee VM, Detre JA, Borthakur A, Reddy R. In vivo measurement of glutamate loss is associated with synapse loss in a mouse model of tauopathy. NeuroImage, 2014;101:185-192.
3. Snyder, J. and A. Wilman, Field strength dependence of PRESS timings for simultaneous detection of glutamate and glutamine from 1.5 to 7T. Journal of magnetic resonance, 2010. 203(1): p. 66-72.
4. Open source VeSPA suite: https://scion.duhs.duke.edu/vespa/
5. Duarte JM, Lei H, Mlynarik V, Gruetter R. The neurochemical profile quantified by in vivo 1H NMR spectroscopy. NeuroImage, 2012;61(2):342-362.
Figures