3963
Bilateral functional MRS of GABA with real-time frequency and motion correction at 7T1Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark, 2Philips, Copenhagen, Denmark, 3Center for Magnetic Resonance, Dept. Electrical Engineering, Technical University of Denmark, Lyngby, Denmark
Synopsis
Brain function is largely controlled by inhibitory processes steered by main inhibitory neurotransmitter GABA. In this role, GABA is essential in brain development and plasticity as well as neuropsychiatric and neurodegenerative diseases. In order to accurately measure GABA responsiveness, we designed a bilateral edited fMRS sequence including real-time frequency and motion correction, as the relatively weak GABA signal is highly susceptible to frequency drift and motion. As acquisition of the macromolecule-uncontaminated GABA signal is challenging at lower field strengths, experiments were performed at 7T.
Purpose
The aim of this study is to accurately measure GABA responsiveness by adding real-time frequency and motion correction to a bilateral functional edited MRS sequence at 7T.Introduction
Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the brain and essential in shaping and controlling neurotransmission. Dysfunctional GABA is suggested to be involved in neuropsychiatric and neurodegenerative diseases. The pure GABA signal, i.e. free from macromolecule contamination, is challenging to assess at lower field strengths, but at 7T the uncontaminated GABA signal can be acquired robustly using the MEGA-sLASER sequence [1]. Using a dual-voxel [2] functional paradigm, it is possible to investigate GABA responsiveness in the stimulated brain area as well as contralateral brain area. As the relatively weak GABA signal is highly susceptible to frequency drift [3] and motion, which are likely to occur during a functional paradigm, real-time frequency and motion correction were added to the sequence.
The 3.0 ppm resonance of GABA is coupled to its 1.9 ppm resonance. In Mescher-Garwood (MEGA) editing [4], a refocusing pulse is applied to the coupled resonance in the even acquisitions (edit-on) while in the odd acquisitions J-coupling is not refocused (edit-off). Subtraction of the edit-on and edit-off spectra results in the edited spectrum with a resolved GABA resonance at 3.0 ppm. The co-edited macromolecule resonance at 3.0 ppm can be suppressed by applying refocusing pulses symmetrically around the coupled 1.7 ppm resonance, alternating between 1.9 (edit-on) and 1.5 ppm (edit-off) [5].
Methods
MRS experiments were performed with a 7T MR scanner (Philips, Best, The Netherlands) in combination with a dual transmit coil and a 32-channel receive head coil (Nova Medical, Wilmington, MA, USA). Motion tracking was performed using volumetric 3D EPI navigators [6] that were interleaved before water suppression in every MRS acquisition, using an interleaved scanning framework [7,8]. Acquisitions were discarded afterwards if motion exceeded 1 mm based on the registered navigators. A frequency drift measurement was performed prior to each 3D EPI volume by readouts of the central k-space line.
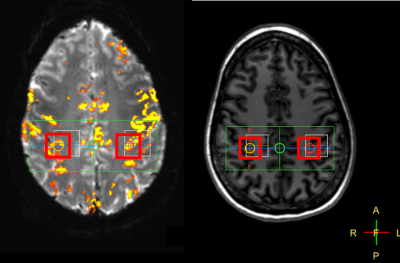
Prior to MRS, T1-weighted MPRAGE (1 mm isotropic resolution, 4 min scan time) and functional MRI (2 mm resolution, 2 min scan time) sequences were performed for voxel placement purposes. To identify the activated brain area, volunteers underwent an fMRI sequence. The task paradigm consisted of three cycles of twenty acquisitions, of which ten acquisitions rest and ten acquisitions of right hand-clenching. Based on the fMRI activation map, 20x20x20 mm3 MRS voxels were placed covering the activated brain area and the contralateral brain area (Fig.1). During fMRS (MEGA-sLASER, 320 acquisitions, TR/TE=3500/74 ms), a rest period of eighty acquisitions was followed by a task period of 160 acquisitions and again a rest period of eighty acquisitions.
Measurements were performed according to the local
ethical protocols.
Results
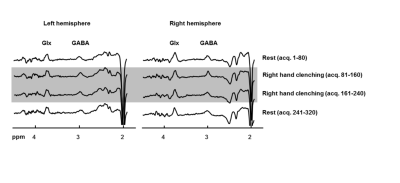
Fig.2 shows edited spectra acquired with real-time frequency and motion correction during rest and task stimulation in the activated brain area and the contralateral brain area. All spectra show a well-resolved GABA resonance at 3.0 ppm.Discussion
Using a bilateral functional edited MRS sequence with real-time frequency and motion correction, the macromolecule-uncontaminated GABA resonance was well-resolved in both voxels and during both rest and task stimulation.Acknowledgements
This research is supported by the Danish Council for Independent Research grant no. 6111-00349A.References
- Andreychenko A, Boer VO, Arteaga de Castro CS, Luijten PR, Klomp DWJ. Efficient spectral editing at 7T: GABA detection with MEGA-sLASER. Magn Reson Med, 2012, 68:1018-1025.
- Harris AD, Glaubitz B, Near J, Evans CJ, Puts NAJ, Schmidt-Wilcke T, Tegenthoff M, Barker PB, Edden RAE. The impact of frequency drift on GABA-edited MR spectroscopy. Magn Reson Med, 2014, 72:941-948.
- Boer VO, Klomp DWJ, Laterra J, Barker PB. Parallel reconstruction in accelerated multivoxel MR spectroscopy. Magn Reson Med, 2015, 74:599-606.
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed, 1998, 11:266-272.
- Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med, 2001, 45:517-520.
- Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, Van der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med, 2012, 68:389-399.
- Henningsson M, Mens G, Koken P, Smink J, Bornar RM. A New framework for interleaved scanning in cardiovascular MR: Application to image-based respiratory motion correction in cornary MR angiography. Magn Reson Med, 2015, 73:692-696.
- Marsman A, Boer VO, Andersen M, Petersen ET. Real-time frequency and motion corrected Hadamard encoded spectral editing (CHASE). Proc Intl Soc Magn Reson Med, 2017, p.5943.
Figures