3961
Simultaneous edited MR spectroscopy of glutathione and macromolecule-suppressed GABA1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Biomedical Engineering, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
γ-aminobutyric acid (GABA) and glutathione (GSH) can be simultaneously measured in the human brain in vivo at 3T using Hadamard encoding and reconstruction of MEGA-edited spectroscopy (HERMES). A drawback of conventional HERMES of GABA/GSH is the contamination of the edited GABA peak with co-edited macromolecular signals (MM), reducing the specificity of the method. We propose the addition of symmetrical suppression into the HERMES framework, and demonstrate the successful implementation of this approach in ten healthy subjects.
Introduction
The main inhibitory neurotransmitter γ-aminobutyric acid (GABA) and the main antioxidant glutathione (GSH) are vital compounds for function and integrity of the human brain. Both metabolites can be detected in vivo at 3T using J-difference-edited MRS techniques such as MEGA-PRESS1,2. However, long acquisition times (~10 min per metabolite per voxel) limit the number of measurements that can be reasonably performed within a clinical MR exam. The finite selectivity of the editing pulses further causes macromolecular (MM) signals to be co-edited with GABA at 3 ppm, potentially amounting to as much as 50% of the observed GABA+MM (‘GABA+’) peak area. This lack of measurement specificity can be addressed by symmetrically arranging two editing pulses around the 1.7 ppm MM resonance3. Hadamard encoding and reconstruction of MEGA-edited spectroscopy (HERMES) is a multiplexed spectral editing scheme allowing simultaneous detection of GABA+ and GSH within a single experiment4. HERMES halves the total scan time relative to sequential measurements of GABA+ and GSH while maintaining the signal-to-noise ratio (SNR) for both edited compounds, but shares the issue of co-edited MM. We therefore introduce MM-suppressed HERMES for simultaneous editing of GSH and GABA without MM contamination.Methods
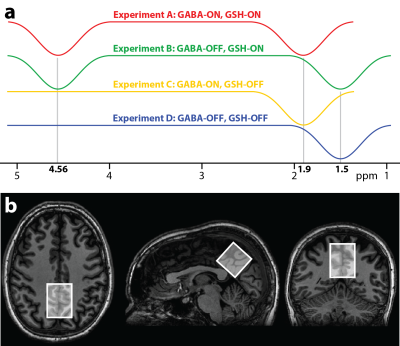
MM-suppressed HERMES contains four sub-experiments (A, B, C and D), with different editing targets (Fig. 1a): (A) 1.9 ppm and 4.56 ppm (ONGABA, ONGSH); (B) 1.5 ppm and 4.56 ppm (OFFGABA, ONGSH); (C) 1.9 ppm (ONGABA, OFFGSH); (D) 1.5 ppm (OFFGABA, OFFGSH). Distinct Hadamard combinations of the sub-spectra give GSH- and MM-suppressed GABA-edited spectra, respectively: (GSH: A + B – C – D) and (GABA: A – B + C – D). To test the proposed scheme in vivo, ten healthy subjects (6 males, mean age 31.3 ± 8.3 y) were recruited with local IRB approval. MRS data were acquired from a 33 × 33 × 33 mm3 midline parietal voxel (Fig. 1b), using a 32-channel phased-array head coil on a Philips 3 T Achieva scanner. Common parameters for MRS scans were TR/TE = 2000/80 ms, 20-ms editing pulses (FWHM = 61.9 Hz), prospective frequency correction5 with 1 water-unsuppressed acquisition per 8 water-suppressed transients. Five MRS scans were acquired per subject: (1) MM-suppressed HERMES (320 averages); (2) conventional HERMES (320 averages); (3) conventional MEGA-PRESS of GABA (160 averages); (4) MM-suppressed MEGA-PRESS of GABA (160 averages); (5) MEGA-PRESS of GSH (160 averages). Data were processed using Gannet6, with frequency-and-phase correction (spectral egistration7 for GABA data, choline-based alignment for GSH data). The 3 ppm GABA resonance and the 2.95 ppm GSH resonances were fit with a single Gaussian model. Metabolite estimates were quantified with respect to the unsuppressed tissue water signal and reported in institutional units (i.u.).Results
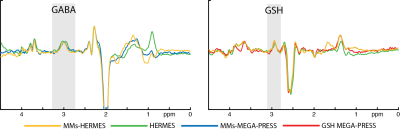
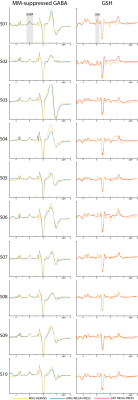
MM-suppressed GABA-/GSH-edited HERMES spectra (orange), conventional GABA-/GSH-edited HERMES spectra (green), and MM-suppressed GABA-edited (blue) and GSH-edited MEGA-PRESS spectra (red) are overlaid for one subject in Fig. 2, with all of the above except the conventional HERMES spectra for all ten subjects displayed in Fig. 3, demonstrating good agreement between single-metabolite and dual-metabolite modalities. Mean GABA estimates were 1.10 ± 0.15 i.u. for MM-suppressed HERMES, and 1.13 ± 0.09 i.u. for MEGA-PRESS (mean difference: -0.03 ± 0.11 i.u.; paired t-test: P = 0.42). The mean GABA/GABA+ ratio was 0.60 ± 0.06 for the HERMES experiments, and 0.67 ± 0.08 for the MEGA-PRESS experiments. Mean GSH estimates were 0.59 ± 0.12 i.u. for MM-suppressed HERMES, and 0.66 ± 0.09 i.u. for MEGA-PRESS (mean difference: -0.07 ± 0.11 i.u.; paired t-test: P = 0.07). A mean SNR improvement of MM-suppressed HERMES over MEGA-PRESS was seen: +1.45 ± 0.25 for GABA and +1.32 ± 0.24 for GSH, i.e., close to the √2 improvement expected from the doubled number of averages compared to the respective separate MEGA-PRESS experiments.Discussion
The qualitative and quantitative agreement between MM-suppressed HERMES and the respective MEGA-PRESS acquisitions indicate successful implementation of symmetrical MM-suppression into the HERMES framework, removing one of the major shortcomings of conventional HERMES of GABA+/GSH.Conclusion
MM-suppressed HERMES allows simultaneous editing of GABA/GSH without macromolecular contamination.Acknowledgements
This work was supported by NIH grants R01 EB016089 and P41 EB015909. NAJP receives salary support from NIH grant K99 MH107719References
1. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–272.
2. Terpstra M, Henry PG, Gruetter R. Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magn Reson Med. 2003;50(1):19-23.
3. Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001;45(3):517-520.
4. Saleh MG, Oeltzschner G, Chan KL, Puts NAJ, Mikkelsen M, Schär M, Harris AD, Edden RAE. Simultaneous edited MRS of GABA and glutathione. Neuroimage. 2016;142:576-582.
5. Edden RAE, Oeltzschner G, Harris AD, Puts NAJ, Chan KL, Boer VO, Schär M, Barker PB. Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. J Magn Reson Imaging. 2016;44(6):1474-1482.
6. Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40(6):1445-1452.
7. Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015;73(1):44-50.
Figures