3958
Iron Oxide-Based T2-weighted MRI and NMR Metabolomics of Radiation and Chemotherapy Induced Inflammation in Glioma Models1Anesthesiology, University of Colorado Denver, Aurora, CO, United States, 2Penn State Health, Hershey, PA, United States, 3University of Colorado Boulder, Boulder, CO, United States
Synopsis
Radio- and chemotherapy for gliomas cause a macrophage-driven inflammation called pseudoprogression (PsP) which appears as abnormal MRI enhancement mimicking treatment response. Our quantitative NMR metabolomics in murine glioma models show a significant increase in amino acid uptake and metabolism (glutamine, glycine, methionine, and tyrosine), lactate and phospholipids in actively proliferating untreated gliomas. Iron oxide is taken up uniquely by inflammatory macrophages, as shown by decreased T2-MRI contrast and an increase in the F4/80 macrophages during treatment-induced PsP. This imaging platform may provide a promising discernment of PsP (iron oxide qT2MRI) and true progression (amino acid PET) for gliomas.
Introduction
The median survival for high-grade gliomas (HGG) after diagnosis is 14.6 months, and the 2-year survival rate is 3.3% [1]. The current standard of care – resection followed by radiotherapy (RT) and concurrent chemotherapy with temozolomide (TMZ) – causes inflammation called pseudoprogression (PS), which appears as abnormal MRI signals at the site of the original tumor within 2-6 months [2]. None of the existing imaging protocols can reliably distinguish PS from true progression in HGG [3]. The purpose of this study was to use NMR-based metabolomics and quantitative T2-weighted MRI (qT2MRI) after injection of super paramagnetic iron-oxide nanoparticles (SPIONs) to mitigate this diagnostic challenge, and to correlate to the in vitro findings. Our working hypothesis is based on the idea that RT+TMZ activated macrophages have a distinct metabolic pattern (as compared to proliferating HGG cells) and will specifically uptake SPIONs resulting in decreased T2-relaxation times of inflamed lesions [4].Methods
In vitro: Murine GL261, human U251 glioma (HGG) cell lines, as well as mouse primary astrocytes and macrophages were cultured using standard cell culture techniques. HGG cell lines were also treated with TMZ and/ or X-ray irradiation. Cell extracts were assessed by 1H- and 13C-NMR spectroscopy followed by the absolute quantification of endogenous metabolites and partial least squares discriminant analysis. In vivo studies involved injecting 10 million U251 and GL261 cells into the right flanks of the female nude athymic (xenograft) and wild-type mice (isograft), respectively. Once the tumors reached a size of 250-300 mm3 mice were treated with oral TMZ daily for 5 days, 10 Gy RT once, or TMZ+RT combination. T2 maps (16 echoes) were acquired followed by injection of ferumoxytol (SPIONs) as a T2 contrast agent. 24 hours later, T2 MRI was repeated. The tumor tissue after the final scan were collected to assess metabolic signatures by NMR, macrophage levels by flow cytometry, and iron levels by a calorimetric assay.Results
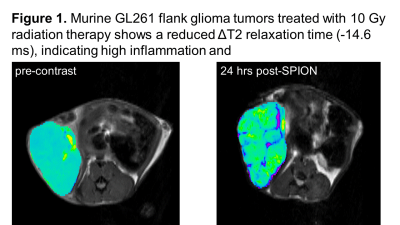
: In vitro: From 75 endogenous metabolites by 1H-NMR, 67% of murine GL261 glioma metabolome differed from primary astrocytes (p<0.05). The HGG-associated changes included a decrease in GABA, myo-inositol, nucleotides, lipids, and a significant increase in phospholipids, lactate and several amino acids (including glutamine, but not glutamate). To validate amino acid metabolism in HGG vs inflammation, 13C-amino acid uptake was assessed in murine HGG cells, primary macrophages and astrocytes. The 13C-NMR studies revealed a highly elevated uptake for glycine, tyrosine, methionine, valine, leucine, and glutamine in HGG cells as compared to normal astrocytes. Inflammatory macrophages had an increased 13C-glutamine uptake only. Highly up-regulated L-type amino acid transporters (LAT1) were also reported in HGG but not in macrophages. Treatment of murine and human HMG cells with TMZ and/ or RT led to a significant decrease of lactate and phosphocholine. A decrease in glucose and amino acid uptake was seen by RT and RT+TMZ only. Ferumoxytol (SPIONs) was taken by macrophages after 4 hrs of incubation, but not by astrocytes or HGG cells. In Vivo: If macrophages take up iron uniquely compared with HGG and normal brain, we expect the SPION T2 signal reduction to be greater with inflammation (PS) which is induced by RT and chemotherapy with TMZ. Indeed, untreated fast-growing HGG tumors showed no changes in T2 relaxation times after injection of ferumoxytol, while irradiated tumors (10 Gy) as well as TMZ treated tumors showed a significant decrease in T2 (up to -15 msec) (Figure 1). When compared with tissue flow cytometry assay, percentage increase in the F4/80 macrophage markers was consistent with the MRI results (10% untreated, 29% treatment). This inflammatory response, as detected by SPION T2-MRI, was more profound in wild-type mice bearing murine GL261 tumors as compare to athymic mice with human U251 xenografts. Metabolically, significantly lower levels of lactate, phospholipids and some amino acids were seen in RT+TMZ treated tumors.Conclusions
Ferumoxytol, an FDA-approved SPION agent, is selectively uptaken by macrophages. It can be successfully used as an MRI contract agent to selectively image an inflammatory response (PS) after both TMZ and RT. HGG signature, on the other hand, can be related to an increased uptake of several amino acids which can be further explored by 18F- or 11C-PET. SPION-based MRI and amino acid PET show a promising discernment of PS from tumor progression non-invasively for HGG.Acknowledgements
No acknowledgement found.References
Alifieris C and Trafalis D. “Glioblastoma multiforme: pathogenesis and treatment.” Pharmacology & Therapeutics, 152: 63-82, 2015.
Chaskis C et et al. “Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations.” Surgical neurology, 72: 423-428, 2009.
Rosenthal CMA. “Imaging modalities in high grade gliomas: Pseudoprogression, recurrence, or necrosis?” Journal of Clinical Neuroscience, 19: 633-637, 2012.
Serkova NJ. “Nanoparticle-based magnetic resonance imaging on tumor-associated macrophages and inflammation.” Frontiers in Immunology, 8: 590-595, 2017.