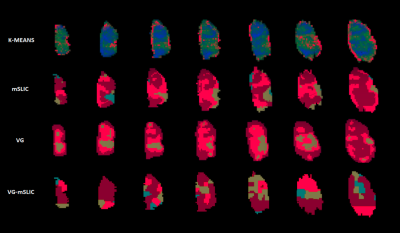3955
Variation-guided supervoxels for subregional tumour analysis in DCE-MRI1Institute of Biomedical Engineering, Department of Engineering Science, University of Oxford, Oxford, United Kingdom, 2Department of Oncology, University of Oxford, Oxford, United Kingdom, 3School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 4Oxford Centre for Functional MRI of the Brain (FMRIB), Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
Synopsis
In nature and real life application domains it is common to encounter varied or textured areas, therefore in many cases it is of greater interest to partition the image into similarly varied, as opposed to similarly homogeneous subregions. We propose a novel, variation-guided approach to SLIC clustering, that has a potential to provide a useful alternative to standard supervoxels due to it’s ability to retain local variation information. We evaluate the method on a longitudinal DCE-MRI dataset of 10 mice scanned over 10 days. The method was able to produce contiguous segmentations, while significantly reducing computational complexity.
Purpose
In tumours, the process of angiogenesis often results in increasingly disorganised and leaky vasculature as tumours progress. This leads to a highly heterogeneous perfusion, which is known to produce both, locally homogeneous and locally varied subregions in tumours.1 Tracking subregional differences, especially the changes in such local variation, might be the key to improve understanding and even predicting tumour progression. Dynamic contrast-enhanced MRI (DCE-MRI) enables monitoring changes in perfusion. Subsequently, image segmentation methods are applied onto DCE-MRI images to define subregions that can be tracked over time.
While there are many methods available for image segmentation, different approaches carry certain assumptions about the distribution of data. Clustering over DCE-MRI parameter maps is often used, however it has been shown to produce noisy segmentations for this application.2 Extracting locally similar areas, or supervoxels (SLIC/mSLIC), 2,3,4 prior to clustering has been shown to improve computational complexity while producing more contiguous segmentations. The method, however, discards valuable information regarding local variation, which might be of crucial importance to segmentation. Patch-based methods 5,6 were previously used to extract locally-varied areas to guide the clustering, however due to their high dimensionality the method is computationally complex. Building on our previous work 3,6 we propose variation-guided mSLIC (VG-mSLIC) supervoxels for subregional tumour analysis, combining the advantages of both methods.
Methods
We evaluate the methods on a dataset of 10 female CBA mice implanted with CaNT tumours scanned over 10 days using 4.7T MRI. T1 mapping was determined from a B1-corrected variable flip angle (VFA) scan with 16 FAs ranging from 1° to 7°. DCE-MRI was performed using cardio-respiratory gated RF and gradient spoiled 3D gradient echo scan (TR 1.4 ms, TE 0.64 ms, FA 5°) with voxel size 0.42x0.42x0.42 mm3.
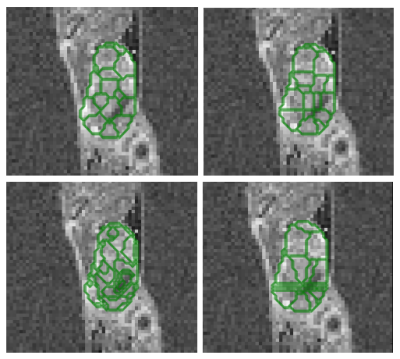
We used principal component decomposition of perfusion enhancement curves to extract three semi-quantitative parameter maps.3 We then apply mSLIC 4 to extract supervoxels (Fig. 1), however, to retain the information about variation we use patches around each voxel instead of raw values to drive the mSLIC clustering. Finally, using average patch values as supervoxel features, K-means clustering is performed across parameter maps to learn a Bag of Visual Words (BOV) model 7 that describes the most dominant visual words, resulting in segmentations.
As no segmentation ground truth was available, we evaluate the segmentations using a contiguity metric, 3 defined as a proportion of 26 neighbouring voxels with a different label to a central voxel:
$$$ C_i = \frac{ | \{ j | l_j \ne l_i \land j \in neigh(i) \} | }{26}, $$$
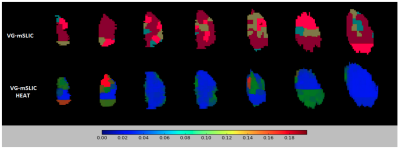
ranging between 0 (contiguous) and 1 (non-contiguous). To evaluate the confidence of K-means clustering over supervoxels, we use confidence heatmaps, defined as the Euclidean distance of each supervoxel from the cluster centre divided by the cluster spread, indicating outliers. Average percentile value for the study is taken ranging between 0 (high confidence) and 1 (low confidence). Finally, we evaluate the segmentations by their power to drive classification of different tumour growth stages.
Results
The VG-mSLIC appears to capture less variance in each feature per supervoxel (93.02% of features with smaller variance), indicating that found supervoxels were similar, while still containing the local variation information lacking in mSLIC. The average contiguity measure for the whole cohort was 0.37 for VG-mSLIC, 0.25 for mSLIC and 0.40 for standard clustering. Although not more contiguous than mSLIC, VG-mSLIC still produced more contiguous segmentations than standard clustering. The average segmentation confidence percentile value for the study was 0.05, indicating that most of the supervoxels were placed close to the cluster centres (Fig. 2). Finally, the growth stage classification accuracy across the study varied between 70% to 83.3% based on VG-mSLIC, as opposed to 83.3% for patch-based method.Discussion and conclusions
Accounting for local variation might be important when deriving meaningful subregions via clustering from physiological imaging of tumours, particularly since different tumours exhibit different homogeneous or heterogeneous properties. Variation guided supervoxels were able to decompose an image into meaningful, locally similar areas, but unlike standard supervoxels, they also retain the valuable information about local variation in perfusion. While there are differences in the distribution of labels between different methods (Fig. 3), our metrics indicated that variation-guided supervoxels are able to produce contiguous segmentations. The classification accuracy based on VG-mSLIC subregions was as good as patch-based. Moreover by significantly reducing the computational complexity, it allows for analysis of multiple parameter maps and efficient application in a challenging environment of heterogeneous tumours. In future we would like to test the usefulness of the method in other domains and applications.Acknowledgements
No acknowledgement found.References
- O’Connor, J., Rose, C., Waterton, J. et al.: Imaging intratumour heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res. 2014; 21(2):249-257.
- Achanta, R., Shaji, A., Smith, K. et al.: SLIC Superpixels Compared to State-of-the-Art Superpixel Methods. IEEE Trans Pattern Anal Mach Intell. 2012. 34(11):2274-2281.
- Irving, B.J., Mirecka, J., Gomes, A.L. et al.: Perfusion- supervoxels for DCE-MRI based tumor subregion assessment. 25th Annual Meeting of ISMRM (2017).
- Irving, B., Popescu, I.A., Bates, R. et al. (n.d.): maskSLIC: Regional Superpixel Generation with Application to Local Pathology Characterisation in Medical Images. arXiv.org. http://doi.org/arXiv:1606.09518
- Varma, M. and Zisserman, A.: A statistical approach to texture classification from single images. International Journal of Computer Vision 62.1-2, 61-81 (2005).
- Mirecka, J., Irving, B., Kannan, P. et al.: Statistical Texture Modeling of Tumour Perfusion Heterogeneity in Dynamic Contrast-Enhanced MRI. Computational Methods for Molecular Imaging, MICCAI (2015).
- Yang, Jun, et al. Evaluating bag-of-visual-words representations in scene classification. Proceedings of the international workshop on Workshop on multimedia information retrieval. ACM, 2007.
Figures