3954
In vivo monitoring of oxygen levels in the brain tumor between fractionated radiotherapy using oxygen-enhanced MR imaging1Department of Medical Imaging, Hefei Cancer Hospital of Chinese Academy of Sciences, Hefei, China
Synopsis
Response of tumor cells to radiation is closely related to oxygen level and fractionated radiotherapy allows reoxygenation of hypoxic tumor cells. Dynamic monitoring of tissue oxygenation is important for precise radiotherapy. Oxygen-enhanced MRI may directly reflect tissue oxygenation, has shown promising applications in the measurement of hypoxia. Therefore, in this study we explored the possibility to monitor oxygen level in the brain between fractionated radiotherapy using oxygen-enhanced MRI. The results showed ΔR1 increased in tumor 30 minutes after first fractionated radiation compared to pre-radiation levels. Thus, oxygen-enhanced MRI can noninvasively monitor oxygen levels in brain tumor between fractionated radiotherapy.
INTRODUCTION
It is well-established that the response of tumor cells to radiation is closely related to tissue oxygen level and fractionated radiotherapy allows reoxygenation of hypoxic tumor cells 1. Dynamic monitoring of oxygen levels in both tumor and surrounding tissue is important for precise radiotherapy against cancer. Recently noninvasively longitudinal relaxation rate (R1)-based MR methods may directly reflect tissue oxygenation in vivo, has shown promising applications in the measurement of tumor hypoxia 2, assessment prostate cancer 3 or radiation-induced necrosis 4. Therefore, in this study we explored the possibility to monitor the changes of oxygen level of tumor and normal tissue in the brain of patients between fractionated radiotherapy using oxygen-enhanced MR imaging.MATERIALS and METHODS
Ethical approval was obtained from the Local Research Ethics Committee. Five patients with single tumor in the brain were recruited and signed informed consents. MR scans were carried out on day 0 (pre-radiation), 30 minutes and 22 hours after first fractionated radiation (i.e. 200 Gy in whole brain). All MR studies were performed on a 3.0 T Achieva scanner (Philips Healthcare, Best, The Netherlands) using an eight-channel SENSE head coil. Anatomical high resolution T2-weighted (T2w) images were acquired in the transverse orientation using Turbo Spin Echo (TSE): TR/TE = 3000/80 ms, TSE factor = 15, field of view (FOV) =230 mm × 184 mm, reconstruction matrix size = 512 × 512, slice thickness = 5 mm. R1 maps were acquired during baseline air breathing using T1 fast field echo (FFE) with variable flip angles (three flip angles from 7 to 37), FOV = 240 mm × 240 mm, in-plane resolution 1.88 mm, slice thickness = 5 mm. Subjects breathed room air to provide baseline data, then 100% oxygen (5 L/min) was delivered through a non-rebreathing facemask for three minutes to ensure stability in blood oxygenation. R1 and ΔR1 (R1 on O2 breathing minus R1 on air breathing) maps were obtained as described previously 3. To quantify ΔR1 changes, each MR dataset was analyzed using ImageJ. Statistical comparisons made between the ipsilateral and contralateral levels were carried out by two tailed Student’s unpaired t tests.RESULTS
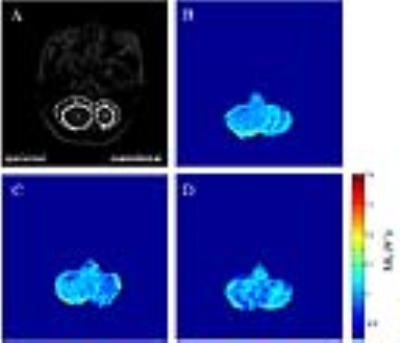
Fig.1 shows that the ΔR1 in the ipsilateral tumor site and normal tissue were smaller than those in the contralateral regions pre-radiation. The ΔR1 increased in the ipsilateral tumor site and normal tissue by 59% and 37%, respectively, 30 minutes after first fractionated radiation compared to pre-radiation levels. Significant recovery of ΔR1 in the contralateral “tumor site” and normal tissue (p < 0.05) were observed 22 hours compared to 30 minutes after radiation levels (0.029±0.007 VS -0.018±0.017, 0.027±0.012 VS -0.014±0.01, respectively).DISCUSSION
The negative ΔR1 in the ipsilateral tumor site and normal tissue suggestive of hypoxia in those regions. After first fractionated radiotherapy, the more positive ΔR1 observed in the tumor site and its surroundings in this study may indicate faster enhancement of tissue oxygen levels in those regions compared to that in the contralateral regions, which may be associated with a large decrease in cell density 5. It may take more time to obtain enough oxygen level in the contralateral normal tissue. In conclusion, oxygen-enhanced MR imaging can noninvasively monitor the changes of oxygen level of tumor and normal tissue in the brain of patients between fractionated radiotherapy.Acknowledgements
This work was supported by the National Natural Science Foundation of China (http://www.nsfc.gov.cn/) grant 81201068.References
1. Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiation Research. 2012;177(3):311-27.
2. Linnik IV, Scott ML, Holliday KF, et al. Noninvasive tumor hypoxia measurement using magnetic resonance imaging in murine U87 glioma xenografts and in patients with glioblastoma. Magn Reson Med. 2014;71(5):1854-62.
3. Zhou H, Hallac RR, Yuan Q, et al. Incorporating Oxygen-Enhanced MRI into Multi-Parametric Assessment of Human Prostate Cancer.Diagnostics (Basel). 2017;24;7(3).
4. Beeman SC, Shui YB, Perez-Torres CJ, et al. O2-sensitive MRI distinguishes brain tumor versus radiation necrosis in murine models.Magn Reson Med. 2016;75(6):2442-7.
5. Lyng H, Sundfør K, Rofstad EK. Changes in tumor oxygen tension during radiotherapy of uterine cervical cancer: relationships to changes in vascular density, cell density, and frequency of mitosis and apoptosis. Int J Radiat Oncol Biol Phys. 2000;46(4):935-46.
Figures