3952
Androgen receptor signaling in castration resistant prostate cancer tumor alters real-time lactate flux and lactate levels in vivo1Urology, University of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Bioengineering, Rice University, Houston, TX, United States, 3Cancer Systems Imaging, University of Texas MD Anderson Cancer Center, Houston, TX, United States, 4Genitourinary Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Non-invasive imaging of castration resistant prostate cancer (CRPC) subtypes remains a challenge in the clinic. CRPC can be subdivided grossly into two phenotypes 1) a morphologically small cell, chemosensitive, and androgen receptor (AR) negative subtype and 2) AR-dependent CRPC characterized by dysregulation of AR signaling. Employing hyperpolarized pyruvate conversion to lactate in vivo as well as lactate measurements ex vivo, we determined the difference in glycolysis between patient derived xenograft (PDX) animal models of these two CRPC subtypes. We have found increased pyruvate to lactate conversion (P <0.04) and higher lactate levels in AR-dependent compared to AR negative PDX models.
Introduction
Androgen receptor (AR) signaling regulated by androgen ligands is one of the most critical pathways for prostate cancer (PCa) pathogenesis and progression. Androgen ablation is used as a first-line PCa therapy. However, patients often succumb to the disease due to PCa progressing from androgen sensitive disease to castration-resistant PCa (CRPC). Molecular analysis of PCa tumors have identified multiple mechanisms that are associated with resistance to AR inhibition therapy including alterations in AR such as amplification, point mutations and/or generation of splice variants in the AR gene that promote development of AR-dependent CRPC disease1-3, in which downstream AR signaling can drive disease in the absence or under low androgen conditions. A clinically aggressive variant PCa (AVPC) subtype that is phenotypically similar to small cell prostate cancer is defined by chemotherapy sensitivity and loss of AR expression and results in the development of AR-independent PCa4. We have developed patient derived xenograft (PDX) animal models that replicate these two CRPC subtypes, which we represent as AR+ or AR-. Our research attempts to link differential metabolic biochemistry associated with alterations in AR signaling in CRPC into a viable imaging biomarker employing hyperpolarized 1-[13C] pyruvate.Methods
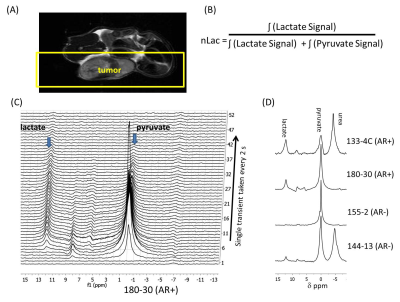
Male SCID mice were implanted subcutaneously with a tumor tissue piece of PDX tumor2,3. Animals were injected with 200 μl of 80 mM hyperpolarized 1-13C pyruvate via tail vein catheter. All imaging and spectroscopy were performed with a 1H volume coil (Bruker BioSpin) with a transmit/receive 13C surface coil placed directly on the tumor in a 7T Bruker Biospec horizontal bore MR scanner equipped with a single channel for carbon excitation/reception. A series of slice-selective 13C spectra were collected right after injection of hyperpolarized 1-[13C]pyruvate. The single slice was placed over the tumor and a total of 90 transients (2s time delay between each transient, 15o - 20o gauss excitation pulse, 2048 data points). The area under the spectral peaks for pyruvate and lactate were integrated over the whole array. Metabolites were harvested from flash frozen tumor tissue and analyzed using both NMR and UPLC-MS/MS. 1D 1H proton spectroscopy was performed with water suppression on a 500 MHz Bruker Avance III HD NMR equipped with a Prodigy BBO cyroprobe. UPLC-MS/MS, chromatographic separation of derivatized tricarboxylic acids, fumarate, succinate, malate and lactate, was conducted using ultra high-pressure liquid chromatography (Agilent 1290 Infinity II), Chromolith C18 reverse phase column (100x2mm, 1.5µm) and a mobile phase gradient from 30% methanol–water with 0.1% formic acid to 95% methanol–water with 0.1% formic acid and, derivatized carboxylic compounds were introduced into the electrospray ion source (Jet Stream, Agilent) and analyzed in the positive ion mode.
Results
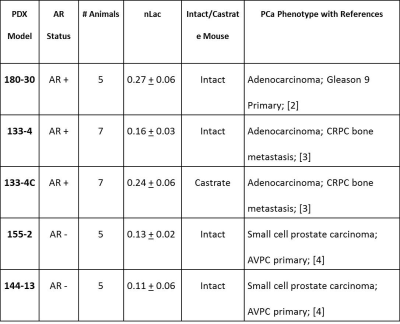
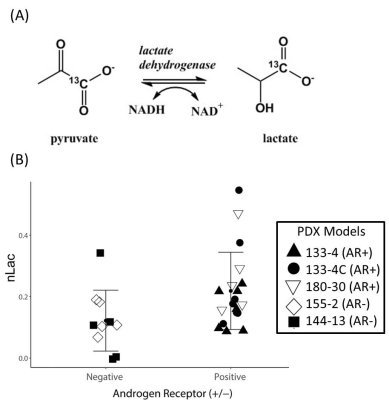
Table 1 summarizes PDX models and PCa phenotype, the number of animals utilized, and the average + standard error of the nLac value. The average relative lactate pool (nLac) (Figure 1B) is calculated as the area under the curve of all transients for lactate over the summed area under the curve from all lactate and pyruvate transient signals. Total nLac in AR+ tumors from intact and castrate mice were greater than 0.15 but both AR- tumors were below 0.15. Statistical significance was observed only when nLac measurements were compared between AR+ and AR- groups (Figure 2B, P value < 0.04 unpaired student’s two-tailed t-test with Welch correction). Hyperpolarized slice-selective magnetic resonance spectroscopy collected from PDX tumors were used to determine lactate to pyruvate ratios (Figure 1).
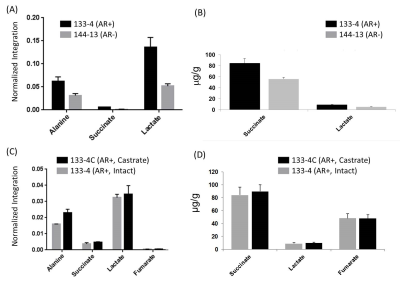
To further confirm our in vivo glycolytic measurements, NMR and UPLC-MS/MS were used to measure metabolites in homogenized tumor tissue samples. We found distinct decreases in lactate and succinate levels both by NMR and UPLC-MS/MS analysis in 144-13 PDX samples relative to 133-4 samples (Figure 3A & B). To evaluate the alterations in glycolytic metabolites after androgen deprivation therapy, the 133-4 PDX was grown in intact and castrate mice as previously described3. The levels of succinate, lactate and fumarate were observed to be similar in both tissue types (Figure 3C & B). These ex vivo measured lactate levels support the observed difference in pyruvate-to-lactate conversion in hyperpolarized-MR of these PDX models.
Discussion
Using preclinical animal models that represent the heterogeneity of CRPC, we found increased glycolysis in androgen signaling PCa compared to AR-negative models. These differences were found in overall lactate values ex vivo found both by NMR and MS analysis and in real time flux measurements (nLac). With the advances in second-generation AR inhibition compounds (abiraterone acetate and enzalutamide), an non-invasive in vivo method to determine if AR signaling is intact even when a patient has undetectable blood androgen levels would help determine if these new agents should be used in a specific patient for neoadjuvant or adjuvant therapy.Acknowledgements
The research was funded in part by a grant from the U.S. Department of Defense (CDMRP PC110065, NZ, SS, JL); by Institutional Research Grants (NMZ, PB); Koch Foundation Genitourinary Medical Oncology Funds (NZ, PB, MT, SM) and a startup grant (PB) from MD Anderson Cancer Center; by grants from the U.S. National Cancer Institute (P50 CA 094056, U54 CA151668, R21CA185536), and by a grant from the Gulf Coast Consortium (JL, PB). This work also was supported by the National Institutes of Health/NCI Cancer Center Support Grant under award number P30CA016672 and used the small animal imaging and the NMR spectroscopy core facilities at MD Anderson Cancer Center.References
1. Yuan X, Cai C, Chen S, Chen S, Yu Z, Balk SP. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene. May 29 2014;33(22):2815-2825.
2. Tzelepi V, Zhang J, Lu JF, et al. Modeling a lethal prostate cancer variant with small-cell carcinoma features. Clinical Cancer Research. Feb 01 2012;18(3):666-677.
3. Maity SN, Titus MA, Gyftaki R, et al. Targeting of CYP17A1 Lyase by VT-464 Inhibits Adrenal and Intratumoral Androgen Biosynthesis and Tumor Growth of Castration Resistant Prostate Cancer. Scientific Reports. Oct 17 2016;6:35354.
4. Aparicio AM, Shen L, Tapia EL, et al. Combined Tumor Suppressor Defects Characterize Clinically Defined Aggressive Variant Prostate Cancers. Clinical Cancer Research. Mar 15 2016;22(6):1520-1530.
Figures