3946
CEST MRI of 3-O-Methyl-D-Glucose uptake and accumulation in brain tumors1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Neurology, Oncology, and Neuroscience, The Johns Hopkins Medicine, and The Hugo W. Moser Research Institute at Kennedy Krieger, Baltimore, MD, United States
Synopsis
Glucose weighted chemical exchange saturation transfer (CEST) imaging has garnered a lot of interest in the past few years as it can be a safe alternative to gadolinium contrast based MRI for tumor diagnosis. 3-O-methyl glucose (3-OMG) is a structural analog of glucose which, because of its apparent non-toxicity and its property to not get metabolized, has been shown to be another promising CEST contrast agent. Here we explore its application as a CEST contrast agent for assessing brain tumors.
Introduction
3-O-methyl-D-glucose (3-OMG) was used in the 80s-90s to study glucose transport across the blood brain barrier (BBB).1 These studies showed that 3-OMG transport is facilitated through the glucose transporters GLUT-1 and GLUT-3 and that it competes with glucose for access to GLUT.2 However, 3-OMG has no metabolic byproduct, unlike 2-FDG and 2DG, which are widely used in PET/MRI.3 3-OMG recently captured the interest of the chemical exchange saturation transfer (CEST) community. It was shown to act as a contrast agent for several breast tumor models in rats using CEST3-4 and for a stroke diagnostic study using the chemical exchange spin lock (CESL) technique.5 Here, we show the feasibility of using 3-OMG for malignant brain tumor detection.Methods
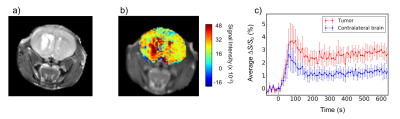
Glioma xenografts of a U87-MG cell line were implanted in five severe combined immune deficiency (SCID) female mice. The tumor growth of 10-15 days was scanned on an 11.7 T Bruker Biospec scanner equipped with a 23-mm volume transceiver coil. Mice were anesthetized by isoflurane in a mixture of O2 and air gases and kept warm with a heating bed. A single intravenous dose of 3 g/kg of 3-OMG was administered for one minute at the rate of 0.2 ml/min. We acquired time-resolved dynamic signals for 3-OMG by saturating at the hydroxyl proton frequency of 1.2 ppm with respect to water, with a temporal resolution of 10 sec and total scan time of 15 min. Saturation was achieved by a single magnetization transfer (MT) pre-pulse (2 s, B1 = 1.5 mT). Imaging was done using a rapid acquisition with relaxation enhancement (RARE) sequence, with repetition time/echo time (TR/TE) = 5.0 s/ 11.15 ms. Pre-and post-infusion CEST Z-spectra were acquired over a frequency range of -6 to 6 ppm.Results and Discussions
The average dynamic enhancement curve (n=5) for the tumor and contralateral brain can be seen from Figure 1c. An elevated uptake of 3-OMG is clear in the tumor, showing a maximum ΔS/S0 = 3.71 ± 0.93 % at 70 s post-injection. ΔS/S0 remains at approximately 2.5% till the end of the scan. The 3-OMG signal intensity in the contralateral part peaks to 2.6 ± 1.0 % and plateaus at 1.1 ± 0.3 % after 200 s. For a similar tumor cell line with glucose infusion, a maximum enhancement of 1.90 ± 0.47 % was observed, which is about half of that for 3-OMG.6Conclusions
3-OMG because of its apparent non-toxicity, transport across the BBB, and a maximum CEST contrast enhancement of about 3-5 %, has potential as a contrast agent for the diagnosis of brain tumors.Acknowledgements
We thank the NIH (Grant RO1 EB019934) for financial support.References
1. Vyska K, Freundlieb C, et al. The assessment of glucose transport across the blood brain barrier in man by use of 3-(11C) methyl-d-glucose. J Cereb Blood Flow Metabol,1981;1: S42–S43.
2. Oldendorf W H, Crane P D, et al. Rapid transient drop in brain glucose after intravenous phloretin or 3-0-methyl-D glucose. Stroke,1983;14:388-393.
3. Rivlin M, Tsarfaty I, Navon G. Functional molecular imaging of tumors by chemical exchange saturation transfer MRI of 3-O-Methyl-D-glucose. MRM, 2014;72(5):1375-1380.
4. Rivlin M, Navon G. CEST MRI of 3-O-methyl-D-glucose on different breast cancer models. MRM, 2017.
5. Jin T, Mehrens H, et al. Chemical Exchange-Sensitive Spin-Lock MRI of Glucose Analog 3-O-Methyl-D-Glucose in Normal and Ischemic Brain. JCBFM, 2017.
6. Xu X, Chan K W Y, et al. Dynamic glucose enhanced (DGE) MRI for combined imaging of blood-brain barrier break down and increased blood volume in brain cancer. MRM,2015; 74(6):1556-1563
Figures