3941
MRI for vitrectomized eyes1C.J. Gorter Center for High-Field MRI, Leiden University Medical Center, Leiden, Netherlands, 2Department of Ophthalmology, Leiden University Medical Center, Leiden, Netherlands
Synopsis
Retinal detachment is a common complication of ocular tumours and is treated by replacing the original vitreous liquid with a silicon oil (SiOil). This SiOil hinders regular ophthalmic imaging, preventing follow-up after radiotherapy. MRI could offer the necessary imaging, but the strong off-resonance of SiOil impedes normal scan protocols. We determined the MR-characteristics of SiOil and developed a corresponding MR-imaging protocol. This protocol was evaluated on an eye tumour patient, who also suffered from retinal detachment. The protocol resulted in high quality MR-images of the eye, allowed a diagnosis of the multiple ocular lesions and averted surgical removal of the eye.
Introduction
Uveal melanoma (UM) is the most common primary ocular tumour. The diagnosis of UM is primarily performed with fundus photography and ultrasound (US), although recent studies have shown the added value of MRI.1,2
One of the common complications of UM is retinal detachment. Retinal detachment is treated with vitrectomy in which the original vitreous fluid is replaced with a silicone oil (SiOil). This oil, however, prevents ultrasound imaging, as ultrasound waves reflect at the SiOil-water interface. This prevents diagnosis and follow-up for these patients, making surgical removal of the eye the only remaining treatment. MRI, however, could potentially provide the necessary imaging data, but the strong off-resonance of SiOil prevents conventional protocols to work. We therefore developed a dedicated protocol to scan the eyes of those patients.
Methods
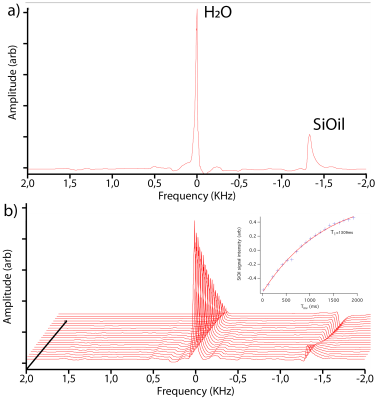
All scans were performed on a 7T Philips Achieva whole body magnet. Initially the MR-characteristics of the SiOil (RS-OIL ECS 1000cS) were determined by scanning a tube consisting of water and SiOil. Single-voxel MR-spectroscopy (PRESS, TE/TR:12ms/3s) was used to determine the resonance frequency of SiOil. Subsequently the T1 of the SiOil was determined by performing an inversion recovery measurement for 20 inversion times (PRESS, TI/ΔTI/TE/TR:20ms/100ms/12ms/10s). These spectra were analysed in jMRUI, using the AMARES-fitting algorithm to determine the signal intensity at each inversion time.
Subsequently an MR-imaging protocol was developed, using a house-build receive eye-coil in combination with a Nova Medical transmit coil. As the eye-coil mainly gets signal from the SiOil, which has a 4.5ppm offset compared to water, a correct calibration and shimming of the MR-scanner is hindered. Therefore, an initial estimate of F0 and power settings is determined by making a low-resolution scan of the complete head with the transmit coil. A MRS voxel was planned on the eye with the same FOV as subsequent imaging scans. The power-, F0 and shim-settings (1st order pencil-beam) from this scan were used for subsequent imaging. The following imaging protocol consisted of T1-weighted scans without any suppression, with SPIR (-1.4kHz) or SPAIR (-1.4kHz,T-inv:700ms) SiOil suppression. As some residual liquid remains in the vitreous, an MPRAGE scan (T-inv:1280ms) with SPIR SiOil suppression was used to supress both the remaining vitreous liquid and SiOil. The protocol furthermore included a T2-weighted scan with SPIR SiOil suppression. In all imaging scans, the cued-blinking paradigm was implemented to minimize eye-motion.
This protocol was developed on a phantom and later used to scan a UM patient twice after giving informed consent. This patient was scanned to screen for potential tumor regrowth after a combination of tumor resection and radiotherapy.
Results
The phantom MR-spectra clearly show the water (4.7ppm) and SiOil (0ppm) peaks, figure 1. An exponential fit of the inversion series resulted in a T1 of 1309ms for the SiOil, figure 1. A comparison between the SPIR and SPAIR SiOil suppression showed good suppression for both techniques.
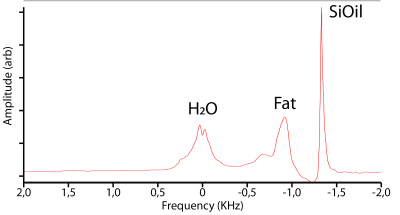
For both the phantom as the in vivo scans, the first attempts to determine the correct F0-, shim- and power settings failed, as for example the F0 determination converged to the wrong spectral peak. This is, however, clearly visible in the resulting spectrum, and was corrected by rerunning the same scan, with a corrected initial F0. Figure 2 shows an in vivo spectrum after correct preparations.
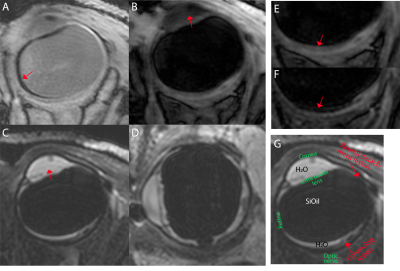
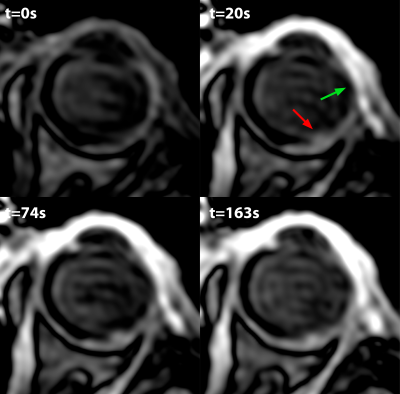
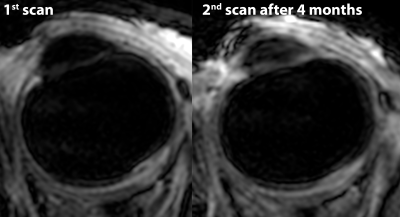
Figure 3 shows a subset of the data from the UM patient. These scans reveal multiple lesions in the eye. The lesions which were located near the retinal detachment did not enhance after gadolinium administration. The lesion on the location were the tumour was previously located did however enhance, figure 4. The patient therefore received a second scan after 4 months, which showed no significant changes compared to the earlier scan, figure 5, suggesting the enhancement was caused by radiotherapeutical damage.
Discussion
Through a modified setup of the MRI, the eyes of vitrectomized patients can be evaluated with MRI. This offers great clinical possibilities, as the SiOil prevents the use of conventional imaging techniques. The presented workflow relies heavily on MRS to determine the correct settings for subsequent imaging. A future integration of these steps in the general imaging preparation would improve the workflow and allow for a wider clinical application of these methods.Conclusion
By enabling MR-imaging of the vitrectomized eyes, new diagnostic options are possible, which can save the eyes of patients with eye tumours.
Acknowledgements
The author thanks M. Versluis (Philips Healtcare, Best, the Netherlands) for stimulating discussions on the adaptation of the scan protocols, and T. Ferreira (LUMC, department of Radiology) for assistance with the interpretation of the MR-images.References
1) Beenakker JWM, et al. Clinical evaluation of ultra-high-field MRI for three-dimensional visualisation of tumour size in uveal melanoma patients, with direct relevance to treatment planning. Magma 29:571-577 (2016)
2) Paul K, et al, Diffusion-Sensitized Ophthalmic Magnetic Resonance Imaging Free of Geometric Distortion at 3.0 and 7.0 T: A Feasibility Study in Healthy Subjects and Patients With Intraocular Masses. Investigative Radiology 50:309 (2015)
Figures