3937
Locally constrained registration of dynamic contrast enhanced MRI time series improves tracer kinetic model voxel-wise fit repeatability in liver tumours1Division of Informatics, Imaging & Data Sciences, University of Manchester, Manchester, United Kingdom, 2Division of Cancer Sciences, University of Manchester, Manchester, United Kingdom, 3CRUK & EPSRC Cancer Imaging Centre in Cambridge and Manchester, Cambridge and Manchester, United Kingdom
Synopsis
DCE-MRI enables the estimation of clinically useful parameters of tissue microvasculature, and is frequently used in trials of anti-angiogenic drugs. However tissue movement can lead to inaccurate parameter estimation. Rapidly changing contrast and limited spatial structure within tumours makes DCE-registration a challenging task. We present a novel algorithm that estimates a model of local tumour motion from the most stable part of the time-series and uses this to constrain registration of the whole series. We demonstrate statistically significant improved extended Kety-model fits and improved parameter repeatability for a set of 59 liver tumours in 40 patients, at two baseline scans.
Introduction
DCE-MRI has been used to assess over 100 trials and investigator led studies of drugs that target angiogenesis[1]. Modelling contrast concentration over a dynamic series enables the estimation of physiologically meaningful parameters[2]. However tissue movement can impair model fitting, leading to inaccurate parameter estimation. In cancer analysis, this is exacerbated for tumours in organs that move significantly and non-rigidly with breathing. While voxel-wise inaccuracies may be “averaged out” using robust whole tumour summary statistics, correcting for motion is necessary when performing sub-volume spatial analysis. Registration of DCE time-series is non-trivial because rapidly changing contrast levels significantly alter image structure, particularly during the main bolus passage phase crucial to accurate model fitting[3,4]. Meanwhile the motion of easy to track large-scale image features (eg organ boundaries, major bones) may not accurately represent a tumour’s local motion. We present a novel algorithm to overcome these challenges, and demonstrate improved model fitting in 59 liver tumours.Methods
Imaging data
40 patients underwent an axial 3D T1-FFE DCE-MRI protocol on a Philips 1.5 T Achieva system (75 time-points; temporal resolution 5 secs; field-of-view 37.5x37.5x10.0cm; spatial resolution 2.93x2.93x4.00mm). Baseline T1 was estimated from using a variable flip angle approach. Contrast agent Gd-DOTA was administered at the 8th time point using a power injector (dose 0.1mMol/kg; flow rate 3ml/s), followed by a saline flush. Patients were rescanned using the same protocol 2-5 days later. 59 tumours with manually delineated ROIs ranging in size from 3.2 to 67.6cm3 were analysed in this work.
Registration
- Volumes are globally aligned to a common reference frame, matching spatial gradients for voxels with the lowest mean absolute change in signal from one time-point to the next. This corrects for bulk changes in patient position, both within series and between visits.
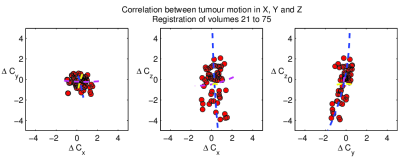
- We compute the mean signal of the 21st to 75th volumes, and rigidly align (allowing rotation/translation but not scaling) each tumour ROI to the mean. These volumes are selected because their contrast levels are relatively stable (compared to main bolus passage phase) but there is enhancement present. We allow maximum translations of ±15mm and rotations of ±15◦ about each axis. These limits can afford to be loose as outliers will not affect the constrained fit applied in step 4.
- The motion of each tumour follows a consistent pattern with breathing, producing strong correlations between the registration parameters (Fig. 1). To recover this pattern we apply principal components analysis (PCA) to the parameters across the 55 selected volumes (Fig. 1). Typically >80% of variation is represented by the first PCA mode, and >95% by the first three.
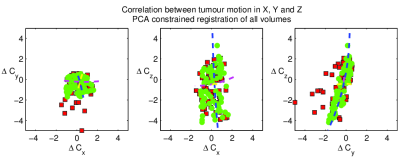
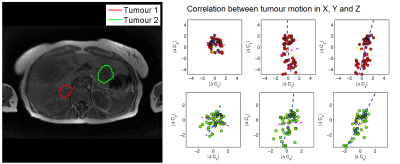
- Finally, all 75 volumes are registered to the mean, optimising parameters for the first three PCA modes, thus constraining motion to the pattern observed in step 3 (Fig. 2). Because breathing motion is non-linear over the liver volume, and thus a tumour’s motion will depend on its location, we repeat steps 2-4 for patients with multiple tumours (Fig. 3). We do not assume where each time point lies in the breathing cycle, as this may vary inconsistently (eg a patient sighing), however we constrain PCA parameters to ±3 standard deviations of their mean.
Model fitting
We fitted an extended-Kety model[2] to both original and registered time-series data in each tumour, computing values of Ktrans, ve and vp, and compared the sum-of-squares error (SSE) of the model fits at each voxel. Additionally, time-series data were converted to contrast concentrations, and used to compute integrated area-under-curve values for 60 seconds post-injection (IAUC60).
Analysis
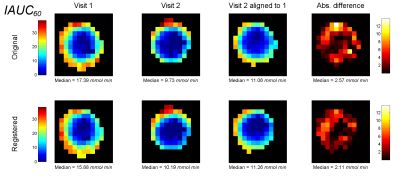
For each tumour in each visit, we tested for changes in model fit SSE after registration using Willcoxon’s signed rank test. We compared repeatability of Ktrans, ve, vp and IAUC60 between visits, testing for changes in median values across the whole tumour, and, following alignment of visit 2 to 1, for individual voxels (Fig. 4).
Results
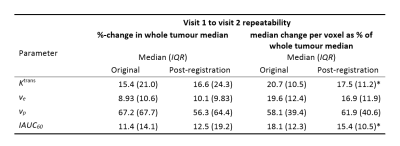
PCA-constrained registration significantly reduced model fit SSE in 58 of 59 tumours for visit 1 and 57 tumours in visit 2. Table 1 summarises changes in parameter estimation between visits. Registration did not significantly change the repeatability of whole tumour parameters, but significantly improved voxel-wise repeatability for Ktrans (p < 0.001) and IAUC60 (p = 0.01).Discussion and conclusion
Our
registration algorithm successfully models local tumour motion and
significantly improved model fit in all but one tumour in the data.
Registration did not improve the repeatability of whole tumour summary
statistics, due to the “averaging-out” effect across the tumour volume. However
comparing parameters at the voxel level showed significantly better matching
after registration, suggesting it is a beneficial step prior to sub-tumour
spatial analysis.Acknowledgements
This work was supported by Cancer Research UK (CRUK) Clinician Scientist award (grant C19221/A22746) and CRUK and EPSRC Cancer Imaging Centre in Cambridge and Manchester funding to The University of Manchester (grant C8742/A18097)References
- O'Connor, JPB et al., Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies, Nature Reviews Clinical Oncology 9, 167–177 (2012)
- Tofts, P. S., Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J. Magn. Reson. Imaging, 7: 91–101 (1997)
- Buonaccorsi GA et al., Tracer Kinetic Model-Driven Registration for Dynamic Contrast Enhanced MRI Time Series. MICCAI LNCS vol 3749 (2005)
- Bhushan M et al., Motion Correction and Parameter Estimation in dceMRI Sequences: Application to Colorectal Cancer. MICCAI, LNCS vol 6891 (2011)
Figures