3936
Evaluation of Pharmacokinetic Models for Perfusion Imaging with Dynamic Contrast-Enhanced Magnetic Resonance Imaging in Skeletal Muscle Using Low-Molecular-Weight Contrast Agents1Klinik und Poliklinik für Strahlentherapie, Universitätsklinikum Essen, Essen, Germany, 2Centre for Cardiovascular Science, Clinical Research Imaging Centre, University of Edinburgh, Edinburgh, United Kingdom, 3Department of Radiation Oncology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 4Department of General and Visceral Surgery, Protestant Hospital Wesel, Wesel, Germany
Synopsis
Pharmacokinetic models for perfusion quantification with DCE-MRI using a low-molecular-weight contrast agent (LMCA) in skeletal muscle of pigs were validated. This in vivo study included compartmental and distributed parameter models which allow estimation of the functional and structural composition of heterogeneously perfused tissues. The different tracer kinetic models, which measure transport parameters in physically meaningful units in heterogeneous tissue, were compared to identify a method that allows reliable quantitative determination of physiological parameters. Double-contrast agent DCE-MRI using LMCA and intravascular CA in combination with the 2-compartment exchange model extended by a nonnutritive arteriolar compartment yields the most reliable results.
Introduction
Pharmacokinetic models for perfusion quantification with dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) using a low-molecular-weight contrast agent (LMCA) in skeletal muscle of pigs were validated. This in vivo study included compartmental and distributed parameter models which allow estimation of the functional and structural composition of heterogeneously perfused tissues (Figure 1). The different tracer kinetic models, which measure transport parameters in physically meaningful units in heterogeneous tissue, were compared to identify a method that allows reliable quantitative determination of physiological parameters such as perfusion, fractional blood volume, and interstitial volume2.
Methods
Contrast agent transport was measured in seven regions of interest placed in the total lower limb supplied by the femoral artery in seven female pigs. DCE-MRI was performed using a 3D gradient echo sequence with k-space sharing. The sequence was acquired twice, first during administration of an LMCA and then after a blood pool contrast agent (BPCA). Signal-time curves were converted into R1-relaxation rate-time curves. Blood flow was augmented by continuous infusion of the vasodilator adenosine into the femoral artery, resulting in an up to four-fold increase in blood flow. Measurements performed with a Doppler flow probe placed on the femoral artery served as ground truth. The results obtained with several LMCA models, such as Tofts model, extended Tofts model, 2-compartment exchange model (2CXM), distributed parameter model (DPM), and tissue homogeneity model (THM), were compared with those of a two-compartment blood pool model (2CBPM) consisting of a capillary and a nonnutritive arteriolar compartment2,3. The three LMCA models were extended by a nonnutritive arteriolar compartment (E2CXM, EDPM, ETHM) and results were compared to 2CBPM and flow probe results as well4. Additionally, fractional blood volume and interstitial volume were validated by histological methods5,6. The models were tested for clinical applicability in a highly vascularized human sarcoma in the lower limb of a 17-year-old female patient using the same MRI technique as described above4.Results
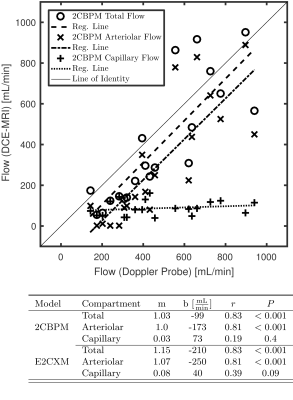
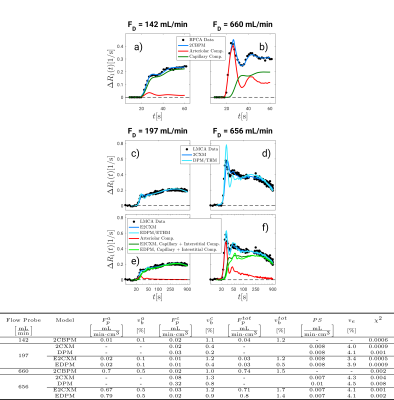
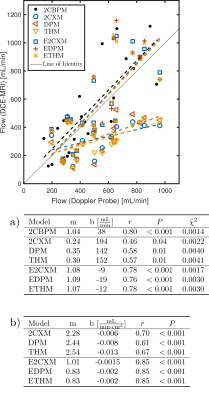
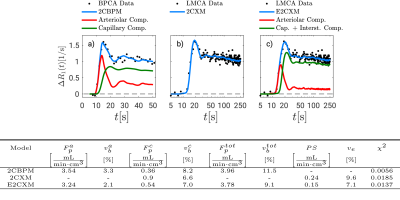
The largest flow increase was found in the arteriolar compartment for all extended models (Figure 2). The two-compartment exchange model extended by an arteriolar compartment (E2CXM) showed the highest fit quality of all LMCA models (Figure 3) and the most significant correlation with the Doppler measurements, r = 0.78 (P < 0.001), see Figure 4. The best correspondence between the capillary perfusion measurements of the LMCA models and those of the 2CBPM was found with the E2CXM (regression line slope of 1, r = 0.85, P < 0.001), see Table b in Figure 4. The LMCA models not extended by an arteriolar compartment deviated markedly from the reference data (Figure 4). In particular, both the simple and the extended versions of the two plug flow models, DPM and THM, yielded unnatural results for the model curve fits to the tissue data (Figure 3). The results obtained with the clinical patient data corresponded very well with the experimental results in pigs4 (Figure 5). Additionally, fractional blood and interstitial volumes were successfully validated by histological methods5,6.Discussion
Our results show that double-CA MRI techniques are necessary for reliable determination of nutritive and nonnutritive perfusion and simultaneous measurement of vascular and extravasation parameters. Considering the physiological condition of opening of nonnutritive shunts by adenosine in our experiments7 and their role in tumor hypoxia1,8 (Figure 1) as well as the high correlation and the very good absolute value assignment in our results, it seems very reasonable to assume that the E2CXM reflects the nonnutritive perfusion which is neglected by simpler models. Although distributed parameter plug flow models account for the uniform CA velocities and path dependence of the CA concentrations in the capillary bed, they neglect varying path lengths. This results in an unnatural discontinuous shape of their residue functions and therefore in a distorted and unnatural appearance of the tissue curve fitting results4 (Figure 3).Conclusions
Distributed parameter models are not appropriate for parameter estimation in skeletal muscle. Double-contrast agent DCE-MRI using an LMCA and a BPCA in combination with the 2-compartment exchange model extended by a nonnutritive arteriolar compartment yields the most reliable results and should be used in clinical routine.Acknowledgements
We would like to thank the Deutsche Forschungsgemeinschaft for their financial support.References
1. Pries AR. European Heart Journal. 2015 Jun; 36(45).
2. Sourbron SP. Physics in Medicine and Biology. 2012 Jan; 57(2).
3. Hindel S. PloS one. 2015 Jun; 10(6).
4. Hindel S. Magnetic Resonance in Medicine. 2017 Oct; Preprint.
5. Hindel S. PloS one. 2017 Jan; 12(1).
6. Hindel S. Investigative Radiology. 2017 Jan; 52(1).
7. Heinonen I. Journal of Applied Physiology. 2010 Feb; 108(2).
8. Pries AR. Nature Reviews Cancer. 2010 Aug; 10(8).
Figures