3934
Multiparametric MRI as a biomarker of response to neoadjuvant second-generation hormone therapy for localized prostate cancer- a pilot study1Radiology, Brigham and Women's Hospital, Boston, MA, United States, 2Dana Farber Cancer Institute, Boston, MA, United States, 3Radiology, Massachusetts General Hospital, Boston, MA, United States, 4Radiology, Children's Hospital Boston, Boston, MA, United States, 5Pathology, Dana Farber Cancer Institute, Boston, MA, United States, 6Pathology, University of Washington, Seattle, WA, United States, 7Oncology, Dana Farber Cancer Institute, Boston, MA, United States
Synopsis
Advanced prostate cancer (PCa) is driven by androgen receptor (AR) signaling, and as such the AR is an important therapeutic target to prevent PCa progression. The aim of this pilot study was to explore a role for prostate multiparametric MRI (mpMRI) as a biomarker for treatment response of localized prostate cancer to neoadjuvant treatment with the second-generation AR inhibitor enzalutamide. We demonstrate that quantitative mpMRI may play an important role as a biomarker of response to neoadjuvant treatment of localized PCa, and MRI-based tumor volumetrics may act as a surrogate for RCB at prostatectomy. These findings are worthy of investigation in a larger clinical setting.
Introduction
Advanced prostate cancer (PCa) is driven by androgen receptor (AR) signaling1-3, and as such the AR is an important therapeutic target to prevent PCa progression4. Given that PCa is a very heterogeneous disease, an individualized approach may be required to maximize potential benefits from novel and combination therapies. A validated metric that measures the optimal length of an individual’s neoadjuvant treatment would be of great benefit in an era where current predictive biomarkers are lacking. The aim of this pilot study was to explore a role for prostate multiparametric MRI (mpMRI) as a biomarker for treatment response of localized prostate cancer to neoadjuvant treatment with the second-generation AR inhibitor enzalutamide.
Methods
Our study was approved by the institutional review board and all patients signed informed consent at screening. Consecutive patients from a single institution with localized prostate cancer (PCa), part of a multi-center prospective randomized open-label phase 2 clinical trial of neoadjuvant enzalutamide either alone or in combination with leuprolide and dutasteride (triple therapy), and for whom radical prostatectomy was indicated, were included. All had a baseline mpMRI and a repeat mpMRI at 3T, after 6-months of neoadjuvant treatment. T2-weighted MRI, apparent diffusion coefficient (ADC) maps, and quantitative pharmacokinetic (PK) parameters from high temporal resolution Dynamic Contrast Enhanced (DCE) imaging were obtained, including AUC90, Ktrans and νe. Tumor regions of interest (TROI) and normal regions of interest (NROI) were volumetrically outlined on all sequences using 3D Slicer (www.slicer.org), and quantitative indices extracted. Pathological staging, residual cancer burden (RCB) and tumor cellularity were measured on prostatectomy specimens. Summary statistics were obtained. Paired t-tests compared TROI and NROI quantitative mpMRI indices, and compared pre-and post-treatment quantitative mpMRI metrics and 3D tumor volumes. To test for correlations between pathology and mpMRI-based RCB, and between mean mpMRI metrics and pathological RCB and cellularity, a Spearman’s rank correlation was used, with Wilcoxon test used to test for differences in volume. Level of statistical significance was set at p<0.05.Results
Eight patients were enrolled, with a mean age of 58.8 ± 7.4 years and a primary Gleason grade 4 on prostate biopsy. Mean PSA nadir was 0.16 ± 0.08 ng/ml. Mean time to PSA nadir was 203.9 ± 25.8 days. At baseline, ADC values were significantly lower in TROIs compared to NROIs, and DCE parameters (Ktrans and AUC90) were significantly higher in TROIs compared to NROIs (Table 1). In response to therapy, this difference between TROI and NROI remained (Table 2), although there was a decrease in Ktrans and AUC90 in areas of TROI and NROIs (p<0.05), and no significant change in ADC metrics. There was a reduction in mean tumor volume based on T2 WI ADC 500, ADC 1400, and DCE (Table 3), all p<0.05. There was no significant difference in post-treatment pathology-based RCB compared to mpMRI-based RCB (based on T2WI, ADC, and DCE), with a strong positive correlation between pathology-based RCB and MR-based RCB (⍴=0.74-0.81, p<0.05) for all sequences. There was a strong positive correlation between pathology-based RCB and post-treatment DCE metrics (AUC90 and Ktrans) and a strong negative correlation with post-treatment ADC metrics (Table 4).Discussion
This pilot study evaluated a role for quantitative prostate mpMRI in assessing response to neoadjuvant therapy, and in characterizing micrometastatic disease. At both baseline and post-treatment mpMRI, we found that quantitative DCE and ADC metrics differ between outlined TROIs and NROIs, and this difference remains after neoadjuvant therapy, despite a significant decrease in the DCE metrics Ktrans and AUC90 in both TROIs and NROIs. This may be explained by the fact neoadjuvant ADT has an effect on all prostate tissue, and the observed changes in DCE parameters are likely reflective of the antivascular effects of ADT. In addition, quantitative DCE metrics and tumor volume measurements from the post-therapy mpMRI strongly correlate with RCB at subsequent prostatectomy. This would suggest that abnormal quantitative metrics may predict the extent of residual functioning tumor, which could be important in terms of early detection of non-responders, and in terms of identifying those who are responding but may benefit from a longer period of treatment, prior to prostatectomy.Conclusion
This pilot study demonstrates that quantitative mpMRI may play an important role as a biomarker of response to neoadjuvant treatment of localized PCa, and MRI-based tumor volumetrics may act as a surrogate for RCB at prostatectomy. These findings are worthy of investigation in a larger clinical setting.Acknowledgements
U01CA151261
P41EB015898
References
1. de Bono JSD, Logothetis CJ, Molina A, et al. Abiraterone and increased survical in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
2. Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983-992.
3. Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.
4. Sternberg CN, Petrylak DP, Madan RA, Parker C. Progress in the treatment of advanced prostate cancer. Am Soc Clin Oncol Educ Book. 2014:117-131.
Figures
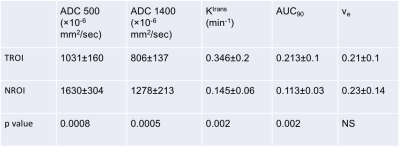
Table 1: Quantitative mpMRI indices at baseline
At baseline, ADC values were significantly lower in TROIs compared to NROIs, and DCE parameters (Ktrans and AUC90) were significantly higher in TROIs compared to NROIs. P value obtained using a paired T test.
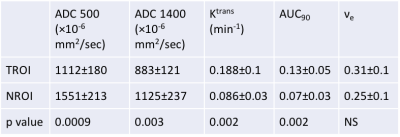
Table 2: Quantitative mpMRI indices after neoadjuvant therapy
After therapy, ADC values remained significantly lower in TROIs compared to NROIs, and DCE parameters (Ktrans and AUC90) remained significantly higher in TROIs compared to NROIs. P value obtained using a paired T test.
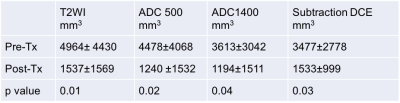
Table 3: MRI-based tumor volume change in response to neoadjuvant therapy
There was a reduction in mean tumor volume, contoured on T2 WI, ADC 500, ADC 1400, and subtraction DCE, using 3D slicer, in response to neoadjuvant therapy.
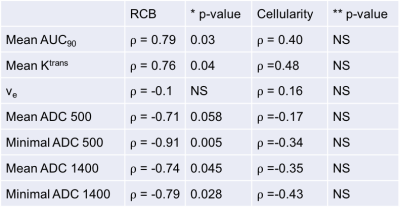
Table 4: Correlations between post-therapy mpMRI quantitative parameters and pathological RCB and cellularity
There was a strong positive correlation between pathology-based RCB and post-treatment DCE metrics (AUC90 and Ktrans) and a strong negative correlation with post-treatment ADC metrics. No correlation was found between quantitative metrics and tumor cellularity