3924
MRS measurement of succinate in vivo as a biomarker in succinate dehydrogenase deficient tumours1Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 2Medical Genetics, University of Cambridge, Cambridge, United Kingdom, 3Endocrinology, Cambridge University NHS Foundation Trust, Cambridge, United Kingdom, 4Pathology, University of Cambridge, Cambridge, United Kingdom, 5Haematology Oncology, University of Cambridge, Cambridge, United Kingdom, 6Diabetes and Endocrinology, UCLH NHS Foundation Trust, London, United Kingdom, 7Medical Oncology, Cambridge University NHS Foundation Trust, Cambridge, United Kingdom, 8Histopathology, Cambridge University NHS Foundation Trust, Cambridge, United Kingdom, 9Immunohistochemistry, Cambridge University NHS Foundation Trust, Cambridge, United Kingdom, 10Radiology, University of Cambridge, Cambridge, United Kingdom
Synopsis
We performed respiratory-gated single-voxel 1H-MRS (TE = 144ms; voxel size 2.2-100ml; 96-512 averages) at 3T in tumours with suspected mutations in the mitochondrial enzyme succinate dehydrogenase (SDH) in 15 patients, analysed using LCModel. A germline mutation or epimutation in one of the SDH genes was identified in 11/15 subjects, with concordant MRS findings in 9 subjects, data rejection as technical failure in 4, and equivocal results in 2. Referencing succinate peaks to choline was an important quality control for discrimination of true from false negatives. MRS may provide a useful biomarker of SDH activity in this patient group.
Introduction
Mutations in the mitochondrial enzyme succinate dehydrogenase (SDH) subunit genes are associated with a wide spectrum of tumors including phaeochromocytoma and paraganglioma (PPGL) 1, 2, gastrointestinal stromal tumours (GIST) 3, renal cell carcinoma (RCC) 4 and pituitary adenomas 5. SDH-related tumourigenesis is believed to be secondary to accumulation of the oncometabolite succinate, but there is a lack of reliable biomarkers to predict tumour aggressiveness and inform on management. MR spectroscopy was recently reported to detect elevated succinate in vivo in patients with SDH deficient PPGL 6. Our aim was to investigate the potential clinical applications of 1H-MRS in a wider range of suspected SDH-related tumours.Methods
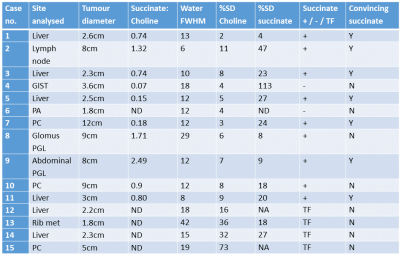
Fifteen patients (9 male; mean age 40 years, range 21-80) were recruited from a dedicated neuroendocrine clinic and a specialist wild-type GIST (wt-GIST) clinic. Single-voxel 1H-MRS (TE = 144ms; voxel size 2.2-100ml; 96-512 averages) was performed at 3T (MR750, GE Healthcare, Waukesha WI) with body coil transmission and reception coils tailored to the tumour location, using respiratory gating if appropriate. Spectral fitting was performed with LCModel 7 using basis sets with simulated peaks of choline, succinate and lipids. Ratios of succinate/choline were reported, and data were classified according to the fitting uncertainty as: 1) technical failure (TF) if choline %SD > 15%; 2) succinate positive if %SD succinate < 50%; 3) succinate-negative if %SD succinate > 50%. An expert spectroscopist also rated whether the identified succinate peak was convincing. Results were correlated with germline mutational status in the four SDHx genes and tumour SDHB immunohistochemistry.Results
A germline mutation in one of the SDH genes was identified in 9/15 subjects; 2 further subjects were diagnosed with a somatic SDHC epimutation (Table 1). A convincing succinate peak was seen in six patients (Table 2), all of whom had a germline SDHx mutation, loss of SDHB by immunohistochemistry, or epimutation in SDHC. Three patients with a tumour choline peak but no succinate peak retained SDHB expression, consistent with SDH functionality. Four cases were identified as technical failures due to uncertainty in fitting the choline peak. In two cases the results were equivocal: Case 7 had a very small succinate peak detected and no mutation found, but insufficient tissue was available for complete analysis; in Case 8 high levels of succinate were reported by LCModel in accordance with the mutation status but the linewidth (29 Hz) was too broad for the result to be deemed reliable. The possible clinical utility of succinate measurement was explored in several cases with follow-up: e.g. in Case 5, the succinate peak increased markedly in size with disease progression (Fig. 1).Discussion
This prospective case series shows that MRS measurement of succinate may be useful as a clinical tool to indicate SDH functionality in patients with endocrine tumours. The clearest results were obtained in non-haemorrhagic liver metastases, where the quality of respiratory-gated spectra was highest, but acceptable quality was also attained in some cases of paraganglioma, abdominal nodes, GIST tumours, phaeochromocytoma, and pituitary adenoma.
Referencing the succinate peaks to choline was an important quality control for discrimination of true from false negatives, which was absent from previous work 6. Choline can be found in most metabolically-active tumours, and its absence or problems in fitting it was taken as evidence that the overall spectral quality was poor, likely due either to insufficient signal, to motion not fully compensated by respiratory triggering or to inability to achieve an acceptable shim (e.g. FWHM was 42 Hz in subject 13, where a bone metastasis was examined). Quantification relative to unsuppressed water was rejected because the water transients might be affected by respiration to a different extent than the metabolites since they were collected separately at the end, and more importantly because, unlike choline, the water signal is not limited to viable tumour tissue but is present and even stronger in areas of necrosis.
Although the widespread clinical application of this technique may be limited by the need for specialist input in the acquisition and interpretation of the data and the inaccessibility to MRS of some tumours due to their location or size, it can in some cases provide useful and specific clinical information which would otherwise be impossible to obtain non-invasively.
Acknowledgements
This study was funded by the Cambridge Experimental Cancer Medicine Centre, Cancer Research UK, and Health Research Board Ireland. The authors would like to thank Stephen Provencher for providing the simulated basis set used in spectral fitting, the radiographers and staff of the MRIS Unit at Addenbrooke’s Hospital and the staff of the Tissue Bank at Addenbrooke’s hospital for assistance, and all the patients who participated in this study.References
1. Astuti D, Latif F, Dallol A, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet 2001;69:49–54.
2. Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma.Science. 2000 Feb 4;287(5454):848-51.
3. Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc. Natl. Acad. Sci. USA 108, 314–318 (2011).
4. Vanharanta S, Buchta M, McWhinney SR, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet 2004;74:153–159.
5. Xekouki P, Stratakis CA. Succinate dehydrogenase (SDHx) mutations in pituitary tumors: could this be a new role for mitochondrial complex II and/or Krebs cycle defects? Endocr Relat Cancer 2012;19:C33–C40.
6. Lussey-Lepoutre C, Bellucci A, Morin A, et al. In Vivo Detection of Succinate by Magnetic Resonance Spectroscopy as a Hallmark of SDHx Mutations in Paraganglioma. Clin Cancer Res. 2016 Mar 1;22(5):1120-9.
7. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993 Dec;30(6):672-9.
Figures