3918
Hydrogen magnetic resonance spectroscopy: a technique for predicting clinical outcome in patients with head & neck squamous cell cancer with locally advanced cervical nodal disease1Centre for medical imaging, University College London(UCL), London, United Kingdom, 2Centre for medical imaging, University College London, London, United Kingdom, 3Dept of medical physics and biomechanical engineering, University college london hospitals, London, United Kingdom, 4Dept of oncology, University college london hospitals, London, United Kingdom, 5Research department of oncology, University college london, London, United Kingdom
Synopsis
Hydrogen magnetic resonance spectroscopy (1H-MRS) is a technically challenging modality. It has the potential to provide specific metabolic information that could guide clinical decision making. In this study we assess feasibility of performing 1H MRS in patients with head and neck squamous cell carcinoma (HNSCC) prior to treatment and explore its correlation with post-treatment outcomes.
Introduction
Head and neck squamous cell carcinoma (HNSCC) remains one of the most debilitating and disfiguring types of cancer. Despite advances and durable treatment responses in other cancers, recurrent disease has been reported in 50% of patients with HNSCC1. Therefore, the ability to predict treatment outcome could be improved if reliable imaging biomarkers could be developed. This would help clinicians stratify patients based on the aggressiveness of the disease. In this study, we assessed tissue metabolites in correlation to treatment outcomes using hydrogen magnetic resonance spectroscopy (1H-MRS) 2,3Methods
Following institutional approval and consent, 35 patients with HNSCC were recruited for 1H-MRS prior to treatment and followed-up following treatment over a 2 year period.
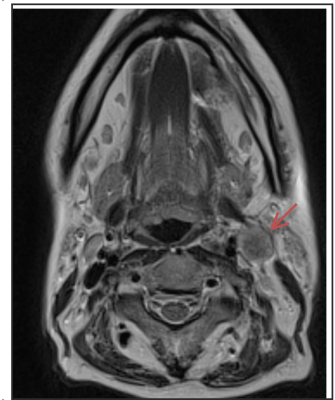
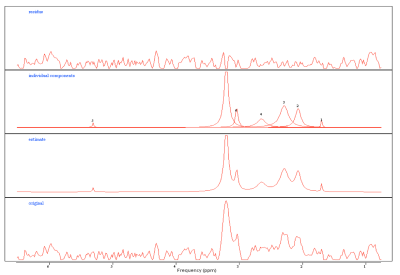
Prior to treatment, all patients were scanned on a 1.5T MR scanner (MAGNETOM Avanto, Siemens AG, Erlargen, Germany) using the carotid coils in the supine position. Anatomical MR imaging across the 3 orthogonal planes was acquired for the 1H-MRS localisation. Manual shimming was performed to ensure acceptable spectral quality and a 15 x 15 x 15 mm3 spectroscopic volume of interest (VOI) was placed over the diseased node (Figure 1). A point resolved spectroscopy technique was used with the following parameters: TE=144ms, TR=2000ms, bandwidth=1000Hz, 1024 points acquiring 56 measurements of 4 averages. When the manual shimming was not possible or the spectral quality was too poor, the spectra were excluded. Post-processing analysis was performed using jMRUI package (Figure 2). The water peaks were used for the phase and frequency corrections, and a Hankel-Lanczos singular value decomposition (HLSVD) filter was utilised to remove water (4.7ppm), methyl (0.9ppm) and methylene (1.3ppm). The data were apodised with a 5Hz Gaussian filter and AMARES fitting method was used to estimate the choline/creatine (Cho/Cr) peaks.
Two medical physicists independently scored the quality of the 1H-MR spectra with a scoring range from 1 to 5. The sum of the scores was calculated and a cut off value of >6 was agreed to indicate minimum quality of spectra to be analysed. Cho/Cr ratios were derived for all analysable datasets.
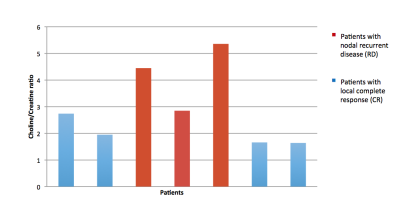
Patients were classified into those with local complete response (CR, n=20) or nodal recurrent/residual disease (RD, n=15) depending on the presence or absence of any local disease relapse based on multi-disciplinary consensus review informed by clinical, imaging and histopathological follow-up. Cho/Cr ratios were compared using an unpaired student t-test between the two groups analysable datasets.
Results
7/35 patients had spectra identified with score of 7 or greater (Figure 3). This was our spectral quality threshold score. Following exclusion of the spectra with lower quality scores of less than 7, the Cho/Cr peak ratios were statistically compared between the two patient groups (number of CR patients = 4 and number of RD patients=3). The mean Cho/Cr ratio of patients within the CR group was (2.0) and was lower than patients within the RD group (4.22) . The comparison of the two groups was statistically significant with a p value of 0.02.Discussion
Our results demonstrate feasibility of using Cho/Cr metabolite ratio as a marker of treatment response. However, we found the technique challenging to apply in the head and neck region – likely related to shimming tolerances of clinical systems 5. Having a minimum of two or more experienced independent scorers allowed us to apply quality control and with the limited number of datasets that passed the quality control threshold we were able to demonstrate significant differences in Cho/Cr ratios between the two patient groups.Conclusion
This study demonstrates the potential clinical value of 1H-MRS as a imaging biomarker as pre-treatment indicator of 2 year treatment outcome in HNSCC. The challenge of obtaining spectra of sufficient quality is highlighted by the high rejection rate during our quality control process. Strategies (preparation/acquisition/post-processing) to improve data quality in the head and neck region will need development before clinical application of Cho/Cr ratios within this patient cohort can be prospectively tested.Acknowledgements
This study was supported by the University College London/King’s College London (UCL/KCL) Comprehensive Cancer Imaging Centre (CCIC) .The work was undertaken at the Biomedical Research Centre (BRC), University College Hospital London (UCLH), which received a proportion of the funding from the National Institute for Health Research (NIHR). This work was also supported by the Royal College of Radiologists UK. The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health.References
1. Pryor DI, Solomon B, Porceddu SV. The emerging era of personalized therapy in squamous cell carcinoma of the head and neck. Asia Pac J Clin Oncol. 2011;7(3):236-51
2. Castillo M, Kwock L, Mukherji SK. Clinical applications of proton MR spectroscopy. AJNR Am J Neuroradiol 1996;17: 1–15
3.Razek, A Abdel Khalek A ; Nada N. Correlation of Choline/Creatine and Apparent Diffusion Coefficient values with the prognostic parameters of Head and Neck Squamous Cell Carcinoma. NMR in Biomedicine, April 2016, Vol.29(4), pp.483-489
4. Rezende TM, de Souza FM, Franco OL. Head and neck cancer: proteomic advances and biomarker achievements. Cancer 2010; 116: 4914–25.
5. Pinker K, Stadlbauer A, Bogner W, Gruber S, Helbich T. Molecular imaging of cancer: MR spectroscopy and beyond. European Journal of Radiology 2012;81:566–77.
6.Tse G, King A, Yu A, et al. Correlation of biomarkers in head and neck squamous cell carcinoma. Otolaryngology: Head and Neck Surgery 2010;143:795– 800.
7. Bisdas S; Baghi M ; Huebner F ; Mueller C ; Knecht, R ; Vorbuchner, M ; Ruff, J ; Gstoettner, W ; Vogl, Thomas J. European Radiology, 2007, Vol.17(1), pp.251-25
Figures