3906
Differences in steady-state glutamate levels and variability between ‘non-task-active’ control conditions: Evidence from ¹H fMRS of the prefrontal cortex1Psychiatry and Behavioral Neurosciences, Wayne State University, Detroit, MI, United States, 2Yale University, New Haven, CT, United States, 3Wayne State University, Detroit, MI, United States, 4Department of Psychiatry and Behavioral Neurosciences, Wayne State University, Detroit, MI, United States, 5Psychiatry and Behavavioral Neurosciences, Wayne State University, Detroit, MI, United States
Synopsis
Proton functional magnetic resonance spectroscopy (1H fMRS) is capable of detecting dynamic changes in brain glutamate related to task engagement compared to a “non-task-active” control condition. The selection of an appropriate control condition is critical, which may confound the magnitude change in glutamate modulation. The purpose of this 1H fMRS study was to compare the steady-state levels of glutamate and its variability in the left dorsolateral prefrontal cortex during four different putative control conditions. Results show significant differences in the glutamate level and variability between conditions, emphasizing the importance of the control condition for the detection of task-evoked glutamate modulation.
Introduction
1H fMRS is a powerful technique for characterizing dynamic changes in brain metabolite levels related to task engagement with a temporal resolution of under a minute. Several recent ¹H fMRS studies have consistently reported elevated glutamate – the primary excitatory neurotransmitter – during neural-activity promoting, ‘task-active’ conditions in focal brain regions, relative to control conditions1,2,3,4. The magnitude and direction of change in the task-evoked glutamate modulation does depend on the comparison “non-task-active” control condition in eliciting a stable, steady-state glutamate level. However, the ‘control’ condition from these studies do vary with respect to constraining behavior and it is unclear whether the acquired steady-state glutamate levels truly reflect a ‘non-task–active’ condition. Using 1H fMRS, this study compared steady-state levels and variability of glutamate in the dlPFC between four different putative control paradigms with the hypothesis that glutamate levels are highly susceptible to the behavioral constraint imposed on the ‘control’ condition.Methods
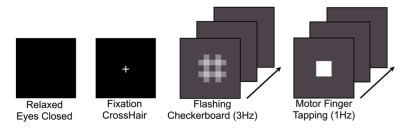
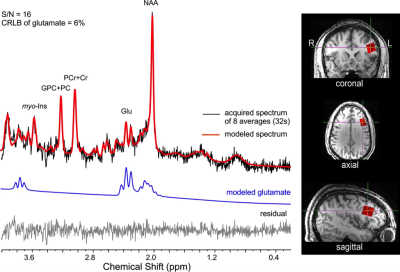
Sixteen healthy adult volunteers (9 males; mean age: 24.3 ± 3.5years), participated in morning ¹H fMRS scanning sessions (9:30-11:30am) using a 3T Siemens Verio system with a 32-channel volume head-coil. Following a structural T1- weighted scan (MPRAGE), a single-voxel (15x20x15mm) was prescribed in the left dlPFC using the AVP approach5, followed by B0-field shimming using FASTESTMAP. Four consecutive ¹H fMRS scans were acquired during different behavioral constraints, including: a) relaxed with eyes closed; b) visual fixation (centered crosshair); c) visual fixation to a flashing checkerboard (3Hz); and d) finger-tapping to periodic stimulus (1Hz; right-hand index finger). The order of conditions b) and d) were counter-balanced. 1H fMRS spectra (N=13) were continuously acquired every 16s (PRESS with OVS and VAPOR, TE=23ms, TR=4.0s, 4 averages/spectrum; 2048 data points; 208s/condition). Prior to quantification, spectra from 2 to 13 were averaged into consecutive pairs to give a 32s temporal resolution (Figure 2). LCModel (v6.3) was used to quantify the metabolite levels, which were expressed in institutional units relative to the unsuppressed water signal. The primary outcome variables of interest were mean glutamate levels and percent coefficient of variation (CV%) from the 6 paired measurements for each condition.Results
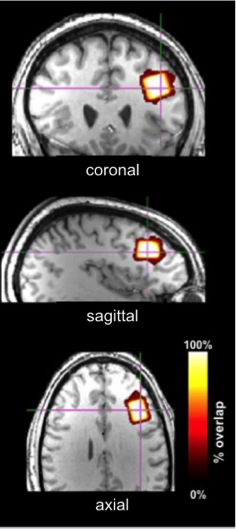
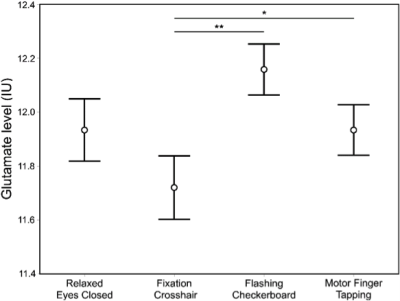
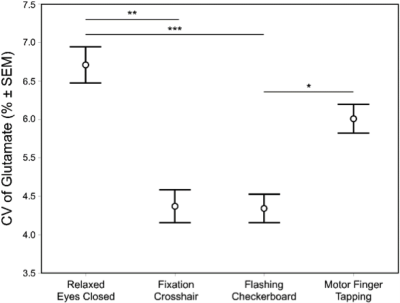
Descriptive data are presented mean ± 1SD. Geometric voxel overlap indicated highly precise (mean % overlap with the template voxel = 92.3±4.7%) and reliable (overlap across all subjects = 88.6%) (Figure 3). Mean voxel grey matter tissue composition was 36.8 ± 3.8%). Mean signal-to-noise (11.7 ± 2.0), full-width half-maximum (4.8 ± 1.0 Hz), and Cramer-Rao lower bound (7 ± 1%) values demonstrated 1H fMRS spectra were fit reliably and did not differ across conditions. Based on a repeated measure analysis, the condition term for glutamate levels failed to reach significance (Χ2 = 5.09; p=.17). However, post-hoc comparisons indicated glutamate levels were significantly lower during the visual fixation crosshair condition (11.6 ± 0.9) compared to both the visual fixation flashing checkerboard (12.2 ± 0.9; p=.0080) and finger-tapping (12.0 ± 0.9; p=.023) conditions (Figure 4). The condition term for the glutamate variability (CV%) failed to reach significance (Χ2 = 6.60, p=.086) (Figure 5). However, post-hoc analyses demonstrated significantly lower glutamate variability during the visual fixation flashing checkerboard compared to both the eyes closed (p=.0037) and finger-tapping (p=.035) conditions. Finally, glutamate levels during the visual fixation crosshair condition were significantly less variable than the eyes closed condition (p=.0087) (Figure 5).Discussion
The motivation of this study was to investigate whether differences in steady-state glutamate levels and its temporal variability in the dlPFC can be detected across four putative ‘control’ conditions. These ‘control’ conditions were chosen because the dlPFC is not the dominant brain region engaged in their implementation. We found mean glutamate levels were qualitatively the lowest during the visual fixation crosshair condition: significantly lower than the flashing checkerboard and finger-tapping conditions. Moreover, the two visual fixation conditions (crosshair and flashing checkerboard) produced the least glutamate variability: significantly lower than the eyes closed condition. The differences in glutamate levels were likely due to the shifting of the excitatory and inhibitory balance (E/I balance) in the brain6,7,8,9 implying that the four ‘control’ conditions differed in their level of engagement of the left dlPFC by shifting the E/I drive. This study provides evidence that steady-state glutamate levels are sensitive to behavioral constraints of ‘non-task-active’ control conditions, and highlights the importance of behavioral paradigms for task-based ¹H fMRS.Acknowledgements
The authors would like to thank Caroline Zajac-Benitez, and the participants in the scanning sessions. This study was supported in part by the Lycaki-Young Funds from the State of Michigan.References
1. Mangia, S., Tkac, I., Gruetter, R., Van de Moortele, P.F., Maraviglia, B., Ugurbil, K., 2007. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1 H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab 27, 1055-1063.
2. Schaller, B., Xin, L., O'Brien, K., Magill, A.W., Gruetter, R., 2014. Are glutamate and lactate increases ubiquitous to physiological activation? A (1)H functional MR spectroscopy study during motor activation in human brain at 7Tesla. Neuroimage 93 Pt 1, 138-145
3. Duncan, N.W., Wiebking, C., Northoff, G., 2014. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans-a review of multimodal imaging studies. Neurosci Biobehav Rev 47, 36-52.
4. Stanley, J.A., Burgess, A., Khatib, D., Ramaseshan, K., Arshad, M., Wu, H., Diwadkar, V.A., 2017. Functional dynamics of hippocampal glutamate during associative learning assessed with in vivo 1H functional magnetic resonance spectroscopy. Neuroimage 153, 189-197.
5. Woodcock, E.A., Arshad, M., Khatib, D., Stanley, J.A., 2017. Automated Voxel Placement: A Linux-based Suite of Tools for Accurate and Reliable Single Voxel Coregistration., Proceedings of the International Society for Magnetic Resonance in Medicine, p. 3022.
6. Isaacson, J.S., Scanziani, M., 2011. How inhibition shapes cortical activity. Neuron 72, 231243.
7. Lauritzen, M., Mathiesen, C., Schaefer, K., Thomsen, K.J., 2012. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. Neuroimage 62, 1040-1050.
8. Maffei, A., 2017. Fifty shades of inhibition. Curr Opin Neurobiol 43, 43-47.
9. Tatti, R., Haley, M.S., Swanson, O.K., Tselha, T., Maffei, A., 2017. Neurophysiology and Regulation of the Balance Between Excitation and Inhibition in Neocortical Circuits. Biol Psychiatry 81, 821-831.
Figures