3903
Assessment of gene therapy efficacy by neurochemical profilingIvan Tkac1, Igor Nastrasil2, Kanut Laoharawee3, Kelly M Podetz-Pedersen3, Kelley F Kitto4, Carolyn A Fairbanks5, Walter C Low6, Karen Kozarsky7, and R Scott McIvor3
1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Dept. of Pediatrics, University of Minnesota, Minneapolis, MN, United States, 3Dept. of Genetics and Cell Biology, University of Minnesota, Minneapolis, MN, United States, 4Dept. of Neuroscience, University of Minnesota, Minneapolis, MN, United States, 5Dept. of Pharmaceutics, University of Minnesota, Minneapolis, MN, United States, 6Dept. of Neurosurgery, University of Minnesota, Minneapolis, MN, United States, 7REGENXBIO Inc., Rockville, MD, United States
Synopsis
Mucopolysaccharidosis type II (MPS II), also known as Hunter syndrome, is a rare X-linked recessive lysosomal disorder caused by defective iduronate-2-sulfatase (IDS). Enzyme replacement is the only FDA–approved therapy available for MPS II, but it does not improve neurologic outcomes in MPS II patients. The 1H MRS data acquired from the hippocampus and cerebellum of untreated and AAV9-IDS treated MPS II mice and heterozygote controls clearly demonstrate that the direct transfer of the missing IDS gene to the CNS at 12 weeks of age prevented neurochemical alternations typical for MPS II at 9 months of age.
PURPOSE
Mucopolysaccharidosis type II (MPS II), also known as Hunter syndrome, is a rare X-linked recessive lysosomal disorder caused by defective iduronate-2-sulfatase (IDS), resulting in accumulation of glycosaminoglycans. Enzyme replacement is the only FDA–approved therapy available for MPS II, but it does not improve neurologic outcomes in MPS II patients 1. Recently we published results of the study that evaluated the effectiveness of adeno-associated virus (AAV) vector mediated gene therapy in a mouse model of MPS II 2. This study focused on the IDS gene expression in CNS and its capability for preventing neurocognitive deficiency. The purpose of this study was to assess the efficacy of AAV-vector mediated gene therapy using 1H-MRS based neurochemical profiling in a mouse model of MPS II.METHODS
The IDS knock-out C57BL/6 mice, a well-established model of Hunter syndrome, were used in this study. The AAV vector serotype 9 expressing human IDS was administrated directly to MPS II mouse brains using intracerebroventricular injection at eight weeks of age 2. In vivo 1H MR spectra were acquired from the hippocampus and cerebellum of AAV9-IDS gene treated MPS II mice (MPS II treated, N = 7), untreated MPS II mice (MPS II, N = 8) and heterozygote littermates (control, N = 8) at 9 months of age. Spontaneously breathing animals were anesthetized with 1.0 – 1.5% isoflurane and the body temperature was maintained at 37oC. The 1H MRS data were acquired at 9.4T using FASTMAP shimming 3 and ultra-short TE STEAM (TE = 2 ms) localization sequence combined with VAPOR water suppression 4. Metabolites were quantified using LCModel with the spectrum of fast relaxing macromolecules (MM) included in the basis set and using unsuppressed water signal as an internal reference (assuming 80% brain water content).RESULTS
The quality of 1H MR spectra acquired from the hippocampus enabled reliable quantification of sixteen brain metabolites (Fig. 1). This number was slightly reduced in the cerebellar data because of intrinsically increased linewidth of spectra acquired from this brain region (Fig. 2). Small but significant concentration differences were observed between untreated MPS II mice and their controls in both studied brain regions (Fig. 1 and 2). Neurochemical profiles of MPS II mice treated with AAV9-IDS showed remarkable similarity to those of control mice. All significant differences in metabolite levels observed in untreated MPS II mice (ascorbate - Asc, glucose - Glc, lactate - Lac in the hippocampus; GABA, glucose, glutamate - Glu in the cerebellum) were normalized in AAV9-IDS treated MPS II mice (Fig. 1 and 2). In addition, even subtle concentration differences between untreated MPS II mice and controls that did not reach the level of significance (such as glutamine (Gln), N-acetylaspartate (NAA) in the hippocampus and phosphocreatine (PCr), myo-inositol (Ins), taurine (Tau) in the cerebellum) were mostly eliminated in the treated group.DISCUSSION
Neurochemical profiles of the hippocampus and cerebellum were substantially different, characterized especially by high Tau in the hippocampus and high total creatine in the cerebellum. The 1H MRS data acquired from the hippocampus and cerebellum of untreated and treated MPS II mice and heterozygote controls clearly demonstrate that the direct transfer of the missing IDS gene to the CNS using intracerebroventricular injection of AAV9-IDS (at 12 weeks of age) prevented neurochemical alternations (at 9 months of age) associated with the neurodegenerative processes observed in this mouse model of MPS II. These results are in good agreement with the gene expression and behavioral results reported in the similar AAV9-IDS treatment model of MPS II 2.CONCLUSIONS
The results of this study demonstrate that the direct delivery of the missing IDS gene into the CNS at young age, using AAV9 vector mediated transfer, was effective to prevent neurochemical alteration associated with MPS II at later age. These results may serve as an additional support for previously reported studies 2 using similar AAV9-IDS gene therapies and indicated this approach appear to be an effective treatment for Hunter syndrome.Acknowledgements
Supported by: NIH grants P41 EB015894, P30 NS076408, P01 HD032652 and WM KECK FoundationReferences
1. Whiteman DA, Kimura A. Development of idursulfase therapy for mucopolysaccharidosis type II (Hunter syndrome): the past, the present and the future. Drug Des Devel Ther 2017;11:2467-2480. 2. Laoharawee K, Podetz-Pedersen KM, Nguyen TT, Evenstar LB, Kitto KF, Nan Z, Fairbanks CA, Low WC, Kozarsky KF, McIvor RS. Prevention of Neurocognitive Deficiency in Mucopolysaccharidosis Type II Mice by Central Nervous System-Directed, AAV9-Mediated Iduronate Sulfatase Gene Transfer. Hum Gene Ther 2017;28(8):626-638. 3. Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med 2000;43(2):319-323. 4. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 1999;41(4):649-656.Figures
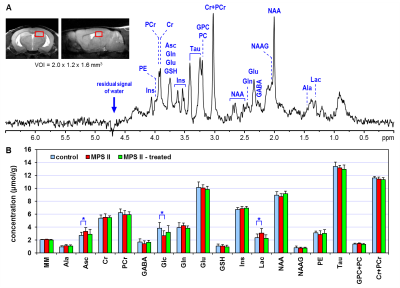
Figure 1 (A) Representative in vivo
1H MR spectrum acquired from the hippocampus of AAV9-IDS treated MPS
II mouse at 9 months of age. STEAM (TE = 2 ms, NT = 320), axial and sagittal
FSE images (thk = 0.7 mm) show the selection of 3.8-µL VOI in the dorsal
hippocampus. (B) Hippocampal
neurochemical profiles of AAV9-IDS treated MPS II mice (MPS II - treated, N = 7),
untreated MPS II mice (MPS II, N = 8) and heterozygote littermates (control, N
= 8). T-test, * p < 0.05, error bars indicate SD.
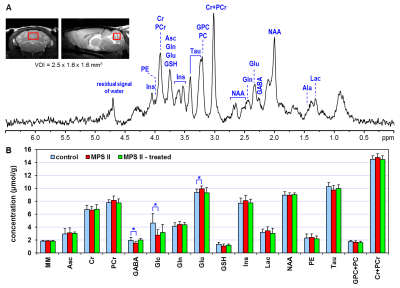
Figure 2 (A) Representative in vivo
1H MR spectrum acquired from the cerebellum of AAV9-IDS treated MPS
II mouse at 9 months of age. STEAM (TE = 2 ms, NT = 320), axial and sagittal
FSE images (thk = 0.7 mm) show the selection of 6.4-µL VOI in the cerebellum. (B) Celebellar neurochemical profiles
of AAV9-IDS treated MPS II mice (MPS II - treated, N = 7), untreated MPS II mice
(MPS II, N = 8) and heterozygote littermates (control, N = 8). T-test, * p <
0.05, error bars indicate SD.