3902
Stress Induced bioenergetic perturbations in CMS rat model of Depression – An invivo phosphorousMRS study at 7T.Hemanth Kumar B S1, Dinesh K Deelchand2, Sushanta Kumar Mishra1, Sadhana Singh1,3, and Subash Khushu1
1NMR Research Centre, DRDO-INMAS, New Delhi, India, 2Center for Magnetic Resonance Research, Department of Radiology, University of Minnesota Medical School, Minneapolis, MN, United States, 3Department of Anesthesiology, University of California Los Angeles, Los Angeles, CA, United States
Synopsis
A chronic mild stress (CMS) animal model for depression was developed and validated using behavioural studies like OFT, FST and SCT. Following anaesthesia, invivo Phosphorous MRS was acquired covering the entire brain using ISIS pulse sequence at field strength of 7T. The spectra thus acquired were processed using LC-model for bioenergy metabolites quantification. The overall study provides new evidence on brain energy related metabolites and redox balance in CMS rats as compared to controls, suggesting that compromised energy metabolism and altered NAD biology observed in CMS rats. The study also revealed changes in High energy phosphate metabolites and membrane phospholipids.
Introduction:
Day to day life stress has shown to play critical role in the etiology of numerous psychiatric illnesses, amongst which depression is a complex psychiatric disorder characterized by anhedonia and feelings of sadness and its etiology is not fully understood1. Chronic Mild Stress (CMS) seems to be a valuable animal model of depression, based on its resemblance with human depressive symptoms2. Advancement in neuroimaging technology helps us to understand the patho-physiology of depression. Phosphorous magnetic resonance spectroscopy (31P-MRS) is a non-invasive neuro imaging technique using which the bioenergitic changes can be measured.Aim:
The present study aimed to investigate the bioenergy metabolite changes in CMS rat brain using invivo 31P-MRS at a magnetic field strength of 7T.Methods:
CMS animal model was developed by applying mild stressors for a period of 6 weeks and was validated using behavioural studies like sucrose consumption test, forced swim test and open field test. Later, following anaesthesia, invivo 31P-MRS was performed in both control and CMS rats (n=10 each) covering the entire brain. MRS was carried out on 7T Bruker biospec (AVANCE III) horizontal bore scanner. Radio frequency (RF) excitation was accomplished with standard double tuned 31P/1H surface coil. MRI protocol included turbo RARE T2-weighted and 31P-ISIS sequence. The first and second order localized shimming was performed using field map based shimming (MAPSHIM), a full-width half-maximum line width of water signal of ≤12 Hz was achieved. In vivo unlocalized 31P NMR spectra (without 1H decoupling) were acquired from the whole brain using a ISIS pulse sequence with an repetition time (TR) of 2.5 s, No. of Averages of 64 and ISIS no of averages of 512 and a slice thickness of 8 mm. A voxel size of 8 X 11 X 12 mm3 covering the entire brain was placed containing only brain tissue, avoiding partial volume effects.Spectral Processing and LCModel Analysis:
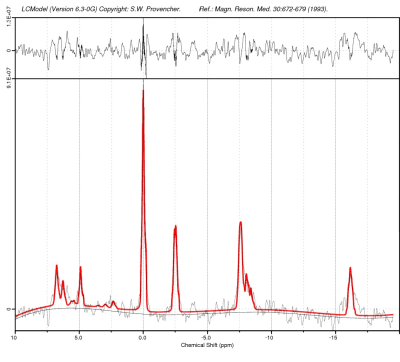
All 31P spectra were processed using Topspin 2.1 (Bruker Biospin, Germany). Spectra from each animal were manually phased (zero- and first-order correction). The spectrum was then referenced by placing the PCr peak at 0 ppm and were analyzed using LCModel (Stephen Provencher Inc., Oakville, ON, Canada) using the MRspa interface in MATLAB (https://www.cmrr.umn.edu/downloads/mrspa/). 31P basis sets were simulated in MATLAB using J-coupling and chemical shift values3 and consisted of 13 basis spectra: PCr, α-ATP, β-ATP, γ-ATP, Pi, nicotinamide adenine dinucleotide (reduced form, NADH and oxidized form, NAD+), phosphorylethanolamine (PE), phosphorylcholine (PC), glycerol-3-phosphorylethanolamine (GPE), glycerol-3-phosphorylcholine (GPC), membrane phospholipids (MP) and 2,3-diphosphoglycerate (DPG). The LC-Model fit for metabolites was fixed with a Crame´r–Rao lower bound (CRLB) of 20% or less.Results and Discussion:
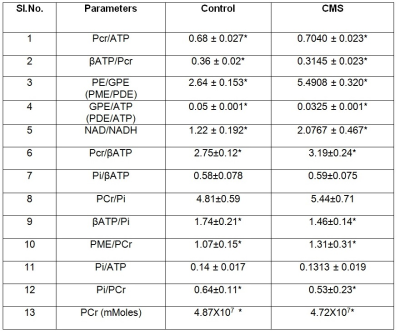
In all the spectra, Phosphocreatine (PCr) spectral intensity was used as the internal reference for relative quantitation due to its relatively stable concentration in the brain. The results showed a decrease in various metabolites like β-ATP/PCr, β-ATP/Pi, GPE/ATP, and Pi/PCr values in CMS rats as compared to control animals. Decrease was also observed in Pi/ATP also but did not attain a statistical significance. Similarly, we observed an elevation in PCr/ATP, PME/PCr, PCr/β-ATP, PE/GPE, NAD+/NADH metabolites in depressed animals as compared to controls. The phosphomonoester (PME) peak contains the signals from numerous metabolites, including metabolites related to membrane phospholipid synthesis, such as phosphocholine (PC) or phosphoethanolamine (PE), and sugar phosphates hence the increase in PME levels in CMS rats suggests that there might be an increased membrane phospholipid turnover in the depressed animals. Increased PCr/Pi ratios depict extensive oxidative phosphorylation in CMS rat brain as compared to control animals. Reduction in β-ATP/PCr, PCr and Pi/PCr levels in CMS rats suggests fluctuation in high energy phosphate that can serve as a substrate of many biochemical processes taking place in brain. The increase in NAD+/NADH concentration in CMS rats suggest their crucial roles in redox reactions related to energy metabolism and antioxidant activity4. The level of NAD+/NADH informs us specifically on oxidative state, these brain metabolites are all at the intersection of multiple critical biochemical pathways within cells, necessary for mitochondrial function and energy metabolism. The increase in PME/PDE levels reflects the membrane degeneration in brain. It has been reported that the PDE peak decrease is caused by hydrolysis of GPC and GPE by activation of phospholipase D5.Conclusion:
Overall, this study provides new evidence on brain energy related metabolites and redox balance in CMS rats as compared to controls, suggesting that compromised energy metabolism and altered NAD biology are observed in those vulnerable to psychosis. The study also revealed changes in High energy phosphate metabolites and membrane phospho- lipid metabolites.Acknowledgements
This work was performed as a part of DRDO sponsored R&D project INM 311 (4.1). The authors are grateful for the support from DRDO, Ministry of Defence, India.References
- Caspi A et. al., (2003), Science 301:386–389.
- Willner P et. al., (2005), Neuropsychobiology 52(2):90-110.
- Deelchand D K, Henry PG., (2015), NMR Biomed. 2015 June ; 28(6): 633–641.
- Kim, S.Y., Cohen, B.M., Chen, X., Lukas, S.E., Shinn, A.K., Yuksel, A.C., Li, T., Du, F., Ongur, D., (2017). Schizophr. Bull. 43 (1), 197–204.
- Li SJ, Prost RW, Tan SG (1993), Proceedings of the Society of Magnetic Resonance in Medicine 1993; 65.