3901
Feasibility of the in vivo measurement of acetate metabolism by 1H-[13C] MRS at 14.1T in the mouse hypothalamus1Laboratory for Functional and Metabolic Imaging, Ecole Polytechnique Féderal de Lausanne, Lausanne, Switzerland, 2Instituto de Investigaciones Biomedicas "Alberto Sols" CSIC-UAM, Madrid, Spain, 3University of Lausanne, Lausanne, Switzerland, 4University of Geneva, Geneva, Switzerland
Synopsis
Astrocytic metabolism is impaired in many central nervous system diseases, like obesity or diabetes. Using 1H-[13C] MRS together with [2-13C]acetate infusion in a 14.1T magnet, we have measured the incorporation of 13C labeling in the mouse hypothalamus in vivo, and calculated its metabolic fluxes using a two-compartment metabolic model that distinguishes between neurons and astrocytes. We think that these results open the possibility of investigating local variations of astrocytic metabolism in the mouse hypothalamus in vivo.
INTRODUCTION
Astrocytes are the most abundant glial cells of the central nervous system. In many central nervous system (CNS) pathologies, such as during obesity or Alzheimer's, astrocytes undergo a process called astrocytosis, which is characterized by an increase in astrocytes' number, changes in molecular expression and morphology and scar formation.
13C
MRS has been previously used to follow the incorporation of [2-13C]Ace in the
rat brain in vivo. In a typical 13C MRS-acetate experiment, acetate, infused trough the femoral vein, is transported across the blood brain
barrier, where is taken up by astrocytes and metabolized into acetyl-CoA, which enters the
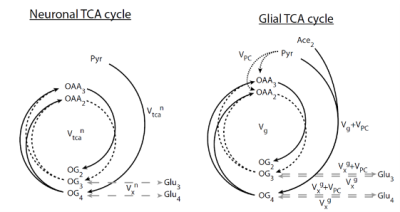
glial tricarboxylic acid (TCA). By infusing labelled [2-13C] Ace, we
can follow the incorporation of the 13C into the TCA products (Gln
C4 and C3, Glu C4 and C3), and infer the neuro-glial metabolic fluxes2 (Figure 1). In small areas, however, 13C MRS experiments using labelled acetate are very challenging, since 13C-labelled signal intensities are small.
On these grounds, the aim of the study was to demonstrate the feasibility of 1H-[13C] MRS techniques to investigate astrocytic metabolism in the mouse hypothalamus, by infusing [2-13C]acetate, evaluating the turnover curves of its metabolic products and calculating the local metabolic fluxes.
METHODS
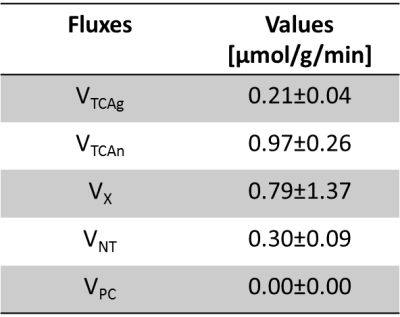
C57BL/6 mice (12weeks, n=5) were scanned in a 14.1 T/26cm magnet (Varian, Palo Alto) and using a 1H-13C coil (11mm 1H loops in quadrature, 10mm linear 13C coil). 1H-[13C] MRS was performed using BISEP-SPECIAL1 (TE/TR=2.8/4000ms) on a 8.7 µL (1.8x2.7x1.8) voxel containing the mouse hypothalamus. Field homogeneity was improved using first and second order shimming with FAST(EST)MAP4., and water suppression with OVS and VAPOR5. [2-13C] acetate infusion was performed with two initial bolus (bolus1=3mL/kg (5min), bolus2=1.83mL/kg (5min) and at a posterior constant infusion rate of 6mL/kg/h. BISEP-SPECIAL was acquired with blocks of 16 scans (2min 11s each). Resulting non-edited and edited spectra were added every 18 min, yielding to 8-9 time points per animal. Metabolites quantification was performed using LCModel and after summing the spectral intensities of the five animals for each time point. Results were fitted to a 2-compartment model2 (Figure 1) using MATLAB (Version 2013b, The MathWorks, Inc., Natick, MA, USA), and values for the hypothalamic glial TCA cycle (VTCAg), neuronal TCA cycle (VTCAn), pyruvate carboxilase (VPC) and neurotransmission (VNT) fluxes were obtained.RESULTS
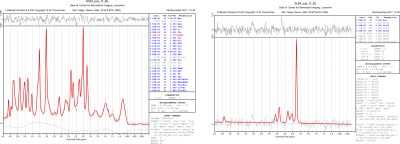
Summed non-edited and edited spectra of the mouse hypothalamus (Figure 2) were obtained with a signal-to-noise (SNR) of 25±2 for the non-edited, and between 11 and 15 for the edited (increasing with the time course of acetate infusion), as measured from LCModel.
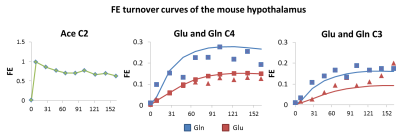
Fractional enrichment (FE) turnover curves of glutamate (Glu) C4 and C3, glutamine (Gln) C4 and C3 and Ace C2 (Figure 3) were used to fit the two-compartment model and the corresponding metabolic fluxes (Figure 4).
CONCLUSIONS
We can conclude that is feasible to perform dynamic 13C MRS in the mouse hypothalamus in vivo using labelled acetate as a substrate. This study opens the possibility of investigating local astrocyte metabolism in vivo in health and disease.Acknowledgements
Authors thank Dr. Hongxia Lei for her technical support. The work was supported by CIBM of the University of Lausanne and Ecole Polytechnique Fédéral de Lausanne, the Leenaards, Jeantet Foundations Swiss National Foundation (149983).References
1Xin et al. 1H-[13C] NMR spectroscopy of the rat brain during infusion of [2-13C] acetate at 14.1T. Magn Reson Med (2010)
2Lanz et al. In vivo quantification of neuro-glial metabolsimand glial glutamate concentration using 1H-[13C] MRS at 14.1T. J Neurochem (2014)
3Mlynarik, V. et al. Localized short-echo-time proton spectroscopy with full signal intensity acquisition. Magn Reson Med (2006)
4Gruetter, R. et al. Field mapping without reference scan using asymmetric echo-plannar techniques. Magn Reson Med (2000)
5Tkac, I. et al. 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med (2009
Figures