3900
High-Resolution Cardiopulmonary Imaging in Free-Breathing Mice using Hyperpolarized Xenon-1291Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Hyperpolarized gas MRI is a well-established tool for assessing lung structure and function in both humans and large animals. However, its utility in small animal models has been limited to terminal studies, as proper gas delivery requires an MR-compatible ventilation scheme that has thus far only been achievable using a terminal intubation process. In this study, we developed a method for delivering hyperpolarized xenon-129 gas to free-breathing mice. An array of pulse sequences were used to acquire high-resolution gas- and dissolved-phase images of the heart and lung structure.
Introduction
Hyperpolarized (HP) gas MRI is a powerful modality for the assessment of both lung function and structure. While its applications are well established in humans [1] and large animals [2], its utility in small animal disease models requires further demonstration [3]. Additionally, its utility for both longitudinal and cross-sectional studies is currently limited by the difficulty of maintaining proper ventilation and gas delivery, as well as by the necessity of a terminal intubation procedure. Here, we present a simple method for continuously supplying HP Xe-129 gas to free-breathing mice to obtain high-resolution gas- and dissolved-phase images of the heart and lung parenchyma. Images were acquired using an array of MRI pulse sequences during xenon gas delivery. This work demonstrates this HP gas MRI’s ability to acquire a set of representative lung and heart images, potentially widening the application of HP xenon imaging in murine models.Materials and Methods
C57BL/6 and BALB/c mice were placed in a modified cradle consisting of a sealed nose cone (to minimize leakage of HP gas around the animal) and inlet and outlet for gas. Anesthesia was maintained by using 1-3% isoflurane mixed with air (20% O2, 80% N2) flowing at 150 ml/min into the nose cone. Mice were placed in a double-tuned 129Xe/1H coil and inserted into a 9.4T vertical-bore micro-imaging MRI system (Bruker Inc.). Axial and coronal proton T2-weighted fast spin-echo (FSE) images were acquired for localization. Enriched xenon-129 gas was polarized using a prototype commercial optical pumping system (XeBox-E10l Xemed, LLC, Durham, NH) that provided polarizations of 40-50%. Polarized gas was stored in a tedlar bag and placed in a sealed chamber. During imaging, the chamber was gradually pressurized with a syringe pump at a rate of 40 ml/min, and the outflowing xenon gas was mixed with oxygen and air. This yielded a continuous flow of gas to the mouse (30% HP-xenon, 20% oxygen, and 50% nitrogen), which was delivered over 10-15 minutes depending on the volume of HP xenon gas (300-750 mL). Excess gas was expelled through the outlet via a secondary syringe pump. Isoflurane was turned off during xenon delivery to maintain a stable breathing-rate. Once steady breathing was achieved, coronal images were acquired using any of the following pulse sequences: 1) respiratory-gated (one phase-encode per breath) slice-selective gradient echo (GRE) sequence for imaging the gas phase (TE = 1ms, FA = 60°, FOV = 20x20mm2, matrix size = 96x96, four 2.5-mm slices), 2) ultrashort echo time (UTE) sequence to image the dissolved phase via selective excitation (TR/TE = 100/0.1ms, FA = 40°, FOV = 35x25 mm2, matrix size = 128x128, projection), or 3) FID-CSI sequence to image both dissolved and gas phases (TR/TE = 80/0.5ms, FOV = 25x25mm2, matrix size = 64x36, FA = 20°).Results and Discussion
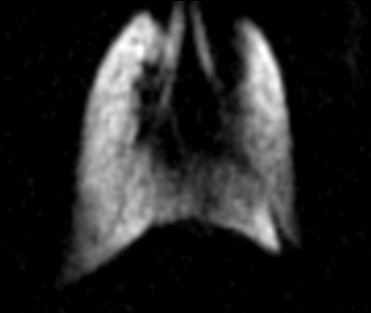
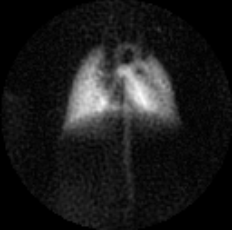
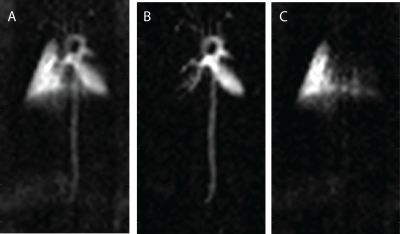
Figure 1 shows a gas-phase image of a mouse lung acquired using a GRE pulse sequence. A clear delineation between the left lobe and right post-caval lobe is visible, as are the lobar and subsequent bronchi. This sequence has consistently produced clear and succinct images of mouse lungs. Figure 2 shows a dissolved phase image of a mouse lung and heart acquired using a UTE pulse sequence. The ultrashort echo times achieved with this sequence enables the acquisition of clear dissolved phase images of the lung and heart structures, which are otherwise unobtainable using the GRE sequence due to the short T2* relaxation time constants of the dissolved phase in mouse lungs at high field strengths. Lastly, Figure 3 shows dissolved phase images obtained from a normal mouse (with a left lung injury) using the FID-CSI pulse sequence. We obtained two images (Fig 3B, 3C) from a single acquisition image (Fig 3A) by fitting two separate Lorentzians to the broad and narrow components of the spectra in each voxel, thereby distinguishing the lung parenchyma (with the short T2*) from the heart and vasculature (with the long T2*). This simple pulse sequence is available on most scanners, but is limited to low spatial resolution due to the considerably long scan times required. Our proposed method enables continuous delivery of HP gas for an extended period of time, which is essential for obtaining high resolution images of the lungs.Conclusion
In addition to demonstrating a simple method for delivering HP xenon gas to free-breathing mice, we used an array of MR pulse sequences to produce high-resolution gas- and dissolved-phase images of the lungs and heart.Acknowledgements
No acknowledgement found.References
[1] Qing, Kun, et al. Journal of Magnetic Resonance Imaging 39.2 (2014): 346-359.
[2] Ruppert, Kai, et al. Magnetic resonance in medicine 51.4 (2004): 676-687.
[3] Wakayama, Tetsuya, et al. Journal of Magnetic Resonance Imaging 27.4 (2008): 777-784.
Figures