3898
Noninvasive biomarkers for the early diagnosis and staging of hepatic fibrosis : A real-time in vivo hyperpolarized 13C MR spectroscopyChung-Man Moon1, Yong-Yeon Jeong2, Il-Woo Park2, and Sang-Soo Shin2
1Advanced Institute of Aging Science, Chonnam National University, Gwangju, Republic of Korea, 2Radiology, Chonnam National University Medical School, Gwangju, Republic of Korea
Synopsis
Hepatic fibrosis associated with chronic liver injury can progress to cirrhosis and ultimately hepatocellular carcinoma. To date, liver biopsy has been regarded as the gold standard for detecting hepatic fibrosis but with practical constraints. Therefore, alternative non-invasive diagnostic methods that can precisely evaluate progression of hepatic fibrosis are urgently needed. However, an in vivo study for hepatic fibrosis using hyperpolarized 13C-labeled pyruvate has not yet been attempted until now. The purpose of this study was to investigate the cellular metabolic changes at different stages of hepatic fibrosis for the early diagnosis.
Introduction
Hepatic fibrosis associated with chronic liver injury can progress to cirrhosis and ultimately hepatocellular carcinoma. To date, liver biopsy has been regarded as the gold standard for detecting hepatic fibrosis but with practical constraints. Therefore, alternative non-invasive diagnostic methods that can precisely evaluate progression of hepatic fibrosis are urgently needed. However, an in vivo study for hepatic fibrosis using hyperpolarized 13C-labeled pyruvate has not yet been attempted until now. The purpose of this study was to investigate the cellular metabolic changes at different stages of hepatic fibrosis for the early diagnosis.Methods
Mild (n = 5) and severe (n = 5) fibrosis were induced in the C3H/HeN mice by injecting thioacetamide (TAA) dissolved in PBS, along with 10% ethanol in water solution, by the intraperitoneal (IP) route three times per week for 15 weeks. Also, C3H/He mice were injected with PBS (7.4 pH) simultaneously by the IP route as normal controls (n = 4). HyperSense DNP polarizer was used to hyperpolarize [1-13C] pyruvate, and the real time 13C MRS and metabolic imaging were performed on the mouse liver following an injection of hyperpolarized [1-13C] pyruvate.Results and Discussion
The levels of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine kinase (CK) and total protein (TP) were not significantly different among the three groups by one-way analysis of variance (ANOVA) test. Fig. 1 shows the stacked 13C MR spectra in each of three stages covering short time periods from 0 to 60 s. More importantly, hepatocellular metabolite levels were significantly different among the three groups (Fig. 2). The ratios of [1-13C] lactate (Lac)/total carbon (tC) and [1-13C] alanine (Ala)/tC were significantly higher in both mild and severe fibrosis groups than in the normal control group (P < 0.05). While the [1-13C] Lac/tC ratio was not significantly different between mild and severe fibrosis, the ratio of [1-13C] Ala/tC was significantly higher in the severe fibrosis than in the mild fibrosis. In addition, Fig. 3 displays each 13C 2D spectroscopic image for hyperpolarized [1-13C] Lac and [1-13C] Ala signals. In hepatic histopathology, compared with the normal control liver (Fig. 4A), the mild fibrosis indicated enlargement of the portal areas by fibrosis, as well as fibrosis extending out from the portal areas with rare bridges between portal areas (Fig. 4B). Also, the severe fibrosis indicated many bridges of fibrosis that link up portal and central areas of the liver (Fig. 4C). From these findings, we assumed that significant increases of [1-13C] Lac and [1-13C] Ala are closely related to the progression of hepatic fibrosis.Conclusion
Our study demonstrated differential patterns of metabolic changes in mild and severe hepatic fibrosis by using a real time in vivo hyperpolarized 13C dynamic MRS and metabolic imaging. The levels of [1-13C] Lac and [1-13C] Ala could be potentially considered as important biomarkers for the early diagnosis and staging of hepatic fibrosis. These findings would be valuable for an understanding of the underlying mechanisms of hepatic fibrosis.Acknowledgements
This work was supported by the fund from the National Research Foundation of Korea (2015R1A2A2A01007827; 2017R1A6A3A11030092).References
Spielman DM, Mayer D, Yen YF, et al. In vivo measurement of ethanol metabolism in the rat liver using magnetic resonance spectroscopy of hyperpolarized [1-13C] pyruvate. Magn Reson Med. 2009;62(2):307-13.Figures

Fig. 1. The stacked 13C MR spectra of the liver, which
were acquired from normal control (A), mild (B) and severe (C) fibrosis-induced
mice.
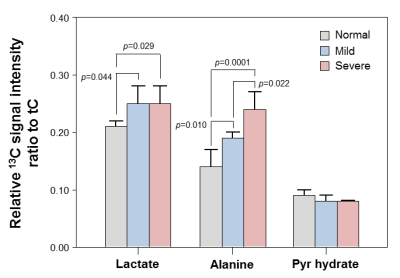
Fig. 2. Cellular metabolite changes in normal, mild and severe
fibrosis groups. The metabolite ratios were significantly different among the
three groups (ANOVA, P < 0.05).
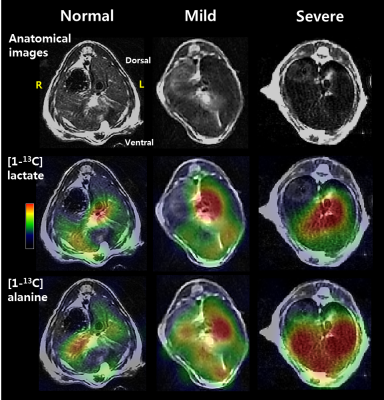
Fig. 3. Representative anatomical images and MR spectroscopic
images of [1-13C] lactate and [1-13C] alanine in a mouse
model with normal control, mild and severe fibrosis in the liver. R = right; L
= left.
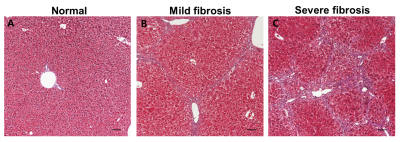
Fig. 4. Typical H&E stained hepatic tissues with normal
architecture (A) and histopathological changes (B and C).