3896
Response to glutaminase inhibition in patient-derived breast cancer xenograft models1Dept. of Circulation and Medical Imaging, NTNU (Norwegian University of Science and Technology), Trondheim, Norway, 2Dept. of Clinical and Molecular Medicine, NTNU (Norwegian University of Science and Technology), Trondheim, Norway, 3Dept. of Pharmacy, Nord University, Bodø, Norway
Synopsis
We used ex vivo 13C HR MAS MRS to determine glutamine consumption and conversion in two patient-derived xenograft models of breast cancer, aiming to identify metabolic differences between a responding (luminal-like) xenograft and a resistant (basal-like) xenograft. CB-839 inhibited tumor growth in luminal-like, but not basal-like, xenograft tumors. Response to treatment was associated with differences in glutamine utilization. Depletion of proline in responding tumors indicate that the effect of glutaminase inhibitors may be associated with metabolic adaption to tumor hypoxia.
Introduction
Glutaminase inhibitors target cancer cells by blocking conversion of glutamine to glutamate, thereby potentially interfering with anaplerosis as well as synthesis of amino acids and glutathione. The drug CB-839 (Calithera Biosciences, CA, US) has shown promising effects in preclinical experiments (1) and is currently undergoing clinical trials in several human malignancies (clinicaltrials.gov). However, response to glutaminase inhibitors is variable (1). Identification of predictive response biomarkers is needed for optimal utilization of CB-839. In this experiment, we used ex vivo 13C high resolution magic angle spinning (HR MAS) MRS to determine glutamine consumption and conversion in two patient-derived xenograft (PDX)models of breast cancer (2), aiming to identify metabolic differences between a responding (luminal-like) xenograft and a resistant (basal-like) xenograft.Methods
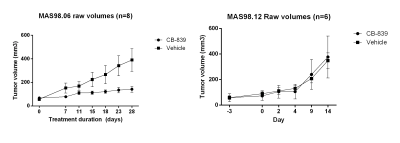
Mice with MAS98.06 (luminal-like) or MAS98.12 (basal-like) tumors received either CB-839 (200 mg/kg) or volume matched drug vehicle two times daily p.o. for up to 28 days (n=6 per group). Tumor size was measured using an electronic caliper two times per week during the experiment.
Another group of mice carrying MAS98.06 or MAS98.12 tumors received either CB-839 (200 mg/kg) or volume-matched drug vehicle two times daily for two days (n=6 per group). Approximately 3 hours after the final dose, [5-13C] glutamine was administered intravenously over three hours (1.2 mg/g body weight). Tumors were harvested and analyzed by HR MAS MRS. Tumors were also harvested from two naïve mice for assessment of the contributions from naturally abundant 13C. Samples were spun at 5kHz and temperature kept at 4°C during the experiments. The following acquisition parameters were applied: One-dimensional 1H noesy pulse sequence with water presaturation (Bruker; noesygppr1d). Acquisition time was 2.7 sec, repetition time 6.7 sec, sweep width was 30ppm, and 128 scans were acquired. 1D 13C MR spectra using a single pulse experiment, with 1H decoupling applied during recycle delay and acquisition (Bruker; zgpg30). The flip angle was 30º, acquisition time 0.9 sec, repetition time was 1.9 sec, sweep width 250 ppm, and 16k scans obtained. Total acquisition time per sample was 9 hours and 45 minutes. The MR spectra were analyzed by principal component analysis (PCA) and the amount of 13C labelled glutamine and glutamate in tumor tissue was evaluated. Immunohistochemistry (IHC) was performed to evaluate expression of glutaminase (GLS), glutamine synthetase (GS), pyruvate carboxylase (PC) and cMYC in tumors from untreated mice.
Results
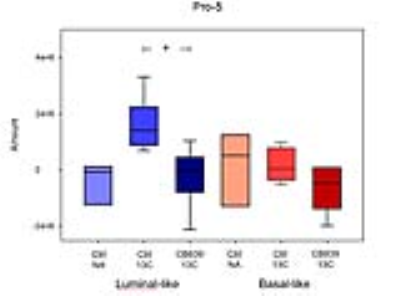
CB-839 significantly inhibited growth in MAS98.06 (p<0.001), but not in MAS98.12 tumors (Figure 1). In untreated tumors, the level of [5-13C] glutamine was similar in both models (Figure 2). However, the ratio of [5-13C] glutamine to [5-13C] glutamate was significantly higher (p<0.05) in luminal-like tumors. In both models, treatment with CB-839 resulted in accumulation of [5-13C] glutamine, consistent with the mechanism of action (Figure 2). In basal-like xenografts, [5-13C] glutamine was converted to [5-13C] glutamate and [1-13C] lactate. In luminal-like xenografts, glutamine was converted to [5-13C] glutamate, [1-13C] alanine and [5-13C] proline. In CB-839-treated luminal tumors, no [1-13C] alanine and [5-13C] proline was detected. No significant differences in expression of GLS, GS, PC or cMyc was observed.Discussion
13C HR MAS MRS described the metabolic fate of [5-13C] glutamine following slow infusion in tumor-bearing mice. Glutamine consumption appeared to be similar in both luminal-like and basal-like tumors. Interestingly, the responding luminal-like xenografts displayed a higher glutamine:glutamate ratio. This could indicate that the two models display a fundamental difference in glutamine utilization, with responding tumors consuming glutamate at a higher rate than resistant tumors. The presence of [1-13C] alanine and [5-13C] proline in the responding tumors further emphasize the difference in glutamine utilization. The effect of CB-839 on glutaminase activity was evident in both a responding and a resistant xenograft model. However, it is noteworthy that CB-839 caused depletion of [5-13C] proline in responding tumors. In contrast to alanine, which can be synthesized through several routes, proline is conditionally essential and glutamate is its main precursor. Recent research has identified proline as an important metabolite in cancer, linking it to adaption to tumor hypoxia (3). The luminal-like MAS98.06 tumors have previously found to be more hypoxic than basal MAS98.12 tumors (4). Our experiment suggests that glutaminase inhibitors may inhibit tumor growth through depletion of proline.Conclusion
CB-839 inhibited tumor growth in luminal-like, but not basal-like, xenograft tumors. Response to treatment was associated with differences in glutamine utilization. Depletion of proline in responding tumors indicate that the effect of glutaminase inhibitors may be associated with metabolic adaption to tumor hypoxia. This warrants further studies of the importance of proline metabolism in response to glutaminase inhibitors.Acknowledgements
CB-839 was provided free of charge from Calithera Biosciences
Animals were housed and treated by the NTNU Comparative Medicine Core Facility.
MRS experiments were performed at NTNUs MR Core Facility
The research was funded by the Norwegian Research Council (grant no: 239940)
References
1. Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13(4):890-901.
2. Bergamaschi A, Hjortland GO, Triulzi T, Sorlie T, Johnsen H, Ree AH, et al. Molecular profiling and characterization of luminal-like and basal-like in vivo breast cancer xenograft models. Mol Oncol. 2009;3(5-6):469-82.
3. Liu Y, Tang L, Zeng J, Geng P, Fang C, Wang Y, et al. Global metabolic profiling identifies a pivotal role of proline and hydroxyproline metabolism in supporting hypoxic response in hepatocellular carcinoma. Clin Cancer Res. 2017.
4. Huuse EM, Moestue SA, Lindholm EM, Bathen TF, Nalwoga H, Kruger K, et al. In vivo MRI and histopathological assessment of tumor microenvironment in luminal-like and basal-like breast cancer xenografts. J Magn Reson Imaging. 2012;35(5):1098-107.
Figures