3894
Partial Normalization of altered brain acetate metabolism in alcohol-dependent subjects after one month of sobriety1Diagnostic Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Psychiatry, Yale University, New Haven, CT, United States, 3Yale Health, Yale University, New Haven, CT, United States, 4Pediatrics, Yale University, New Haven, CT, United States
Synopsis
The purpose of this study is to analyze the adaptation of brain acetate metabolism of AD subjects upon detoxification in order to understand the mechanism of alcohol addiction. Using 13C-magnetic resonance spectroscopy in combination of 2-13C-acetate infusion, and monitor the turn over rates of brain 13C-labeled glutamate/glutamine neurotransmitter, we have found increased brain acetate metabolism after detoxification treatment, suggesting the compromised acetate uptake/metabolism in AD subjects.
Abstract
Background and purpose:
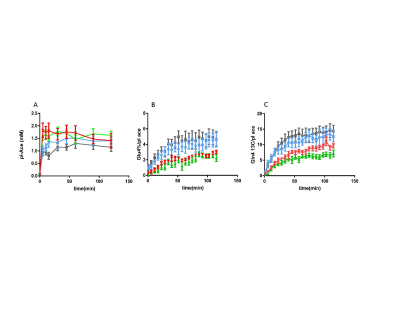
When people drink alcohol, the liver converts ethanol to acetate, which can be used by the brain for energy. Previously we found that healthy non-alcohol dependent heavy drinkers (HD) have increased brain acetate utilization compare to light/non-drinkers (LD). In this study, we evaluated acetate utilization in alcohol-dependent (AD) subjects followed by 3-4 weeks alcohol detoxification/abstinence treatment by analyzing the turnover of glutamate (Glu) and glutamine (Gln) C4 during 2-13C-acetate infusion using 13C magnetic resonance spectroscopy (MRS) in vivo.
Methods:
In this study, 10 AD participants were screened as outpatient with routine laboratory testing, including complete blood count, glucose, BUN/creatinine, electrolytes, folate, B12, liver and thyroid function tests, urine drug screen, urine pregnancy test for females of child bearing potential, and Structured Clinical Interview for DSM-IV Axis-I Disorder (SCID I) (1). After the screening, subjects were admitted to Clinical Neuroscience Research Unit (CNRU) for a one-month medically supervised detoxification and abstinence program. After 4-7 days of abstinence from alcohol, the first 13C MRS with 2-13C-acetate infusion was performed. The subjects remained inpatient for up to one month and underwent a second MRS scan with identical procedures before discharge. We also recruited Heavy Drinkers (HD, n =12), who regularly consumed more than 11 drinks/week + over 3 drinks/occasion at least once a week (for women), and more than 14 drinks/week + over 3 drinks/occasion at least once a week (for men); and Light Drinkers (LD, N=12), who drank less than 3 drinks/week and no more than 2 drinks on any given day. 13C-MR spectra were obtained using localized polarization transfer (PT) with voxel size of 50x40x45 mm on the proximity of occipital cortex, on 4T Bruker ParaVision 6.0 imaging spectrometer. The time courses of spectra obtained in vivo were analyzed with in-house LC-model type software using Matlab. Plasma acetate concentration and 13C-enrichments of Glu and Gln C4 were measured and analyzed using a 500MHz (11.7Tesla) Bruker Topspin 3.2 high-resolution spectrometer. Steady-state levels of metabolites were compared with student t-test.
Results:
8/10 AD participants finished the detoxification program with both 13C MRS sessions. Plasma acetate levels and enrichments in AD showed no significant differences pre- versus post- treatment (p=0.16) but were higher than LD (p=0.017) and HD (p = 0.0002). The 13C enrichments of Glu4 and Gln4 in AD rose significantly after a month of sobriety (p=0.04 and p<0.001, respectively), suggesting increased acetate metabolism after detoxification. Glu4 and Gln4 labeling in the AD early detox (AD1) were significantly lower than in LD (p<0.001 for Glu and Gln) and HD (p<0.001 for Glu and Gln).
Discussion and Conclusion:
The average plasma acetate concentrations in the AD subjects were higher than the LD and HD with the same acetate infusion protocol, suggesting slower systemic consumption of acetate in early sobriety, different from what has been seen in healthy HD. Comparing the 13C-Glu/Gln turnover in non-dependent HD and LD, the AD subjects showed less Glu and Gln labeling in early sobriety versus 1 month. After extended sobriety, Glu and Gln labeling rose to levels comparable to LD/HD. In the brain, acetate is consumed exclusively by glia, so these results suggest that even though non-dependent HD have elevated acetate utilization, AD patients may have impaired glial metabolism in early sobriety.
Acknowledgements
NIAAA R01 AA021984.References
1. First, Michael B., et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute, November 2002.
2. Jiang, L. et al. Increased brain uptake and oxidation of acetate in heavy drinkers, J Clin Invest. 2013; 123(4): 1605-1614.
Figures