3893
Magnetic Resonance Spectroscopy biomarkers predict patient outcome in subacute spinal cord injury1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland, 2Department of Radiology, Swiss Paraplegic Centre, Nottwil, Switzerland, 3Max-Planck-Institute for Biological Cybernetics, Tuebingen, Germany, 4Clinical Trial Unit, Swiss Paraplegic Centre, Nottwil, Switzerland, 5Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany
Synopsis
Spinal cord injury (SCI) is a very heterogeneous disease that makes it difficult to identify a single biomarker during rehabilitation therapy in order to predict the future patient status. In this study, we applied Magnetic Resonance Spectroscopy to examine specific metabolic markers in the pons 10 weeks after injury and correlated them with changes of the clinical status obtained twice during early rehabilitation.
Introduction
Spinal cord injury (SCI) is a very heterogeneous disease that makes it difficult to predict the future patient status. Although van Middendorp et al.1 have assessed a patient’s ability to walk independently after rehabilitation, and Pavese et al.2 have described the prediction of the bladder function after an SCI, no biomarker is available to predict the rehabilitation outcome. Anatomical MRI is widely used and an important tool to describe the site of injury, but it has limited value in predicting the patient’s clinical status. However, magnetic resonance spectroscopy (MRS) provides complementary biochemical information from the neural tissue non-invasively. In this study, we examine metabolites measured once in the pons of subacute SCI patients 10 weeks after injury for the first time and investigate the correlation with alterations of the clinical status, which has been obtained twice (at the time of MR measurement and before discharge at about six months after injury).Method
MRI Protocol & Post-Processing
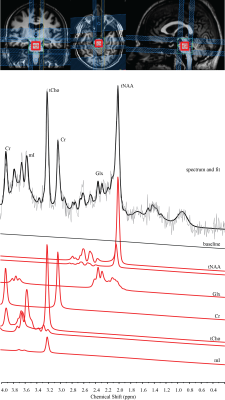
12 SCI patients (age=52.7±12.8 years) participated in this study and had one MR measurement at around 10 weeks (68±10 days) after injury. The measurements were performed on a 3T scanner (Achieva, Philips Healthcare, Best, The Netherlands) using a SENSE head coil. A highly resolved 3D T1-weighted anatomical scan (1x1x1mm3) and a spectroscopic scan including a PRESS sequence with outer-volume suppression was applied (TE=30ms, TR=1600ms). The spectroscopic voxel (25x20x20 mm3) was placed in the pons. All MRS measurements contained 128 signal averages and were fitted using LCModel (Provencher, 1993). The metabolites were quantified individually and the internal water concentration served as reference.
Clinical Assessment
The clinical assessment was conducted twice: 1) around the time of the MR examination (62±14 days after injury) and 2) before discharge (171±74days after injury). The examination included the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) protocol for motor, light-touch and pin-prick scores as described by Kirshblum et al3.
Statistics
The statistical analyses were performed with R (R Core Team, 2016, Version 3.3.1). Normal distribution was checked for each metabolites using the Anderson-Darling test. Correlations between metabolic markers and clinical scores were tested using the Pearson correlation coefficients.
Results
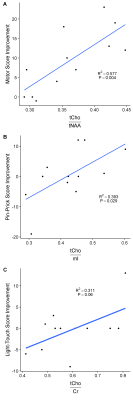
Total N-Acetyl-Aspartate (tNAA), choline containing compound (tCho), total creatine (Cr), glutamate and glutamine (Glx), and myo-Inositol (mI) have been reliably detected (CRLB<25%). Patients showed no significant correlation between the clinical score and the individual metabolic markers. However, the motor score improvements between the neurological scores acquired at two time points (time between two measurements: 108±64 days) significantly correlated with the ratio of tCho/tNAA (R2= 0.577, P=0.004) acquired in the one-time only MR examination. The pin-prick score alterations were positively associated with tCho/mI (R2=0.393, P=0.029). tCho/Cr showed a trend to the light-touch score changes (R2=0.311, P=0.06), although six patients showed no changes (value: 0).
Discussion
We acquired spectroscopic data in 12 SCI patients once 10 weeks after acute injury. The region of interest was the pons, since it serves as an important communication hub between spinal cord, cerebellum and cerebrum. The main results of this study are that two ratios of metabolic markers (tNAA/tCho and tCho/mI) acquired once in the pons significantly correlate with the improvements of motor score and pin-prick in spinal cord injury patients during the following weeks. tCho is a marker for membrane turnover. tNAA is a acetyl donor in myelin sheath development and thus a neuronal marker indicating the viability of the nervous tissue. An upregulation of mI indicates inflammation. Therefore, the correlation of tCho/tNAA with the recovery of motor strength may indicate the importance of increased membrane turnover during rehabilitation. The positive correlation of the ratio tCho/mI with the alterations of the pin-prick score is possibly an indication of increased membrane turnover with decreased inflammation in better performance. These metabolite ratios may reveal underlying adaptation and recovery processes and might therefore be possible state biomarkers. Richard-Denis et al4 have shown in a recent study that among others light-touch and motor score are the best predictors of the Spinal Cord Independence Measure (SCIM) at one year after the incident. Our data might support the prediction of the sensory scores, the SCIM and eventually the patient status.Conclusion
This study shows significant correlations between MRS biomarkers and the neurological status improvements during the following weeks. These metabolic ratios offer new insights into biochemical alterations occurring in tissue undergoing reorganization and adaptation following a spinal cord injury. In conclusion, the sensitivity of brain stem spectroscopy holds the potential for application in therapy monitoring and clinical trials.Acknowledgements
The authors thank all the patients participating in this study. Funding by the Swiss National Science Foundation (Grant Number: 143715), the University of Zurich (Clinical Research Priority Program Multiple Sclerosis) and the European Union (ERC Starting Grant, SYNAPLAST MR, Grant Number: 679927) are gratefully acknowledged.
References
[1] van
Middendorp JJ et al. The Lancet 2011;377:1004-1010.
[2] Pavese C et al. PLOS
Medicine 2016;13(6):e1002041.
[3] Kirshblum SC et al.
Journal of Spinal Cord Medicine. 2011;34(6):535-546.
[4] Richard-Denis A et al. Journal
of Spinal Cord Medicine 2017;1-9.
Figures