3884
Retrospective frequency correction for fluorine (19F) MRS using an external reference1Hoglund Brain Imaging Center, University of Kansas Medical Center, Kansas City, KS, United States, 2Department of Neurology, University of Kansas Medical Center, Kansas City, KS, United States, 3Department of Molecular & Integrative Physiology, University of Kansas Medical Center, Kansas City, KS, United States, 4Division of Clinical Pharmacology, Toxicology, and Therapeutic Innovation, Children's Mercy Hospital, Kansas City, MO, United States, 5Department of Pediatrics, Children's Mercy Hospital, Kansas City, MO, United States
Synopsis
Fluorine MR Spectroscopy (19F MRS) allows in vivo quantification of fluorine-containing antipsychotic and antidepressant drug concentrations in the brain. To detect the low concentration of the drugs (~ 5-30 µM) in the brain, it requires multiple repeated acquisitions to increase SNR and the scan time is relatively long. Therefore, it is important to ensure consistent frequency alignment across the repeated acquisitions. However, MR system instability induces drifts of the scanner frequency, particularly following MR scans with a high gradient duty cycle. Previous frequency correction methods for 1H MRS require internal reference signals, such as under- or un-suppressed water, and cannot be applied to 19F MRS, where the SNR of 19F signals is low at each repeated acquisition. The purpose of this study is to investigate the feasibility of using an external reference for retrospective frequency correction in 19F MRS.
INTRODUCTION
Fluorine MR Spectroscopy (19F MRS) allows in vivo quantification of fluorine-containing antipsychotic and antidepressant drug concentrations in the brain. 1-10 Due to the low drug concentration in the brain (~ 5-30 µM), it is necessary to acquire multiple repeated acquisitions to increase SNR and the scan time is relatively long. Therefore, it is important to ensure consistent frequency alignment across the repeated acquisitions. However, MR system instability induces drifts of the scanner frequency, particularly following MR scans with a high gradient duty cycle. 11,12 Previously proposed frequency correction methods for 1H MRS utilize internal reference signals, such as under- or un-suppressed water. 11-14 However, the same approach of using internal reference signals cannot be applied to 19F MRS, where the SNR of 19F signals is too low to reliably determine the frequency. The purpose of this study is to investigate the feasibility of using an external reference for retrospective frequency correction in 19F MRS.METHODS
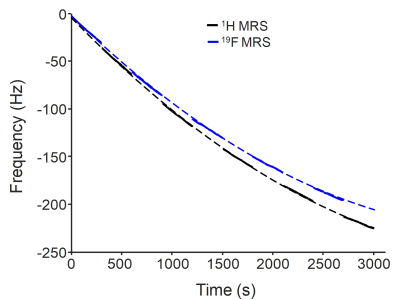
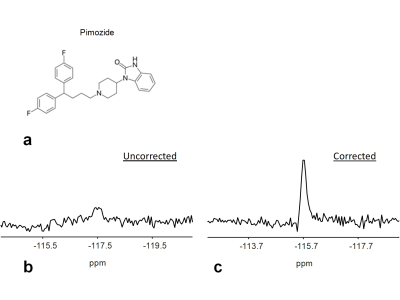
All 19F MRS experiments were performed on a Skyra 3T scanner (Siemens, Erlangen, Germany) using a dual-tuned (1H and 19F) quadrature volume head coil. The calibration of the transmitter power and shimming were performed using 1H measurements and their values were applied to 19F measurements. 19F MRS measurements were performed on a phantom with a similar size (1.5 L) and shape as human head. The phantom contained 0.1 mM pimozide dissolved in dimethylsulfoxide (DMSO). Additionally, a 5 ml cylindrical vial of trifluoroethanol (TFE) was attached to the head coil and was used as an external reference. A non-localized RF pulse of 500 µs was used to excite the 19F signals. The flip angle was adjusted to be the Ernst angle: 60° based on the measured T1 relaxation time of pimozide: 1422 ms on a phantom and TR of 1000 ms. Sequence parameters were TR = 1000 ms, number of samples = 2048, and spectral width = 12 kHz. A 30-min diffusion weighted imaging (DWI) experiment was performed before 19F MRS measurements to create gradient heating-induced frequency drifts. To investigate the behavior of frequency drifts on 19F and 1H, 19F MRS measurements were interleaved with 1H MRS measurements with a total number of averages of 3000 (Fig. 1). Frequency drifts of 19F were determined from the peak position of the spectrum and were measured from the external reference (TFE). Frequency drifts of 1H were measured from the solution phantom (DMSO solvent). Frequency correction for 19F MRS was performed by multiplying the FID signal by a linear phase term. The linewidth and area under the curve (AUC) of the pimozide spectrum were evaluated.RESULTS
Frequency drifts of 19F and 1H MRS measurements exhibited a similar temporal variation (Fig. 1), but frequency drifts of 19F were smaller (19F: 6.2 Hz/min versus 1H: 6.5 Hz/min). The differences resulted from the difference in their gyromagnetic ratios: γ19F = 40.05 MHz/T versus γ1H = 42.58 MHz/T. Without frequency corrections, the spectrum from the averaged pimozide signals showed a broad linewidth of 0.4 ppm and a frequency drift of 1.8 ppm (Fig. 2b). The AUC was 7.8 × 10-5. With the frequency correction using the external reference, the linewidth was reduced to 0.2 ppm (Fig. 2c). The AUC was increased to be 1.9 × 10-4.DISCUSSION
Detection of the low concentration fluorine-containing drugs in the brain using 19F MRS requires a long scan time. This study demonstrated that gradient heating-induced frequency drifts during the long scan time resulted in degraded spectral quality and reduced AUC, which are associated with reduced detection sensitivity and increased errors in quantification of drug compounds. The external reference has been used in previous 19F MRS studies as a reference for chemical shifts and as a standard for normalization to reduce the effects of different coil loading. 2,4,7,8,10 This study demonstrated that the external reference can also be used to correct frequency drifts retrospectively, and that those effects of frequency drifts were notably reduced (Fig. 2). Gradient-induced frequency drifts are spatially invariant (Fig. 1). However, frequency drifts resulting from subject motion are spatially varying, and the correction would require an internal reference. For a dual-tuned coil, 1H signals may be used as an internal reference to correct frequency drifts induced by gradient heating and subject motion in 19F MRS, although many practical issues need to be resolved. In conclusion, this study demonstrated the feasibility of using external reference for retrospective frequency correction in 19F MRS that is essential for consistent detection sensitivity and accurate quantification of fluorine-containing drug compounds.Acknowledgements
This study was partly supported by NIH (UL1TR000001, P20GM103418). The Hoglund Brain Imaging Center is supported by the NIH (S10RR029577) and the Hoglund Family Foundation.References
[1]. Bartels et al., Psychiatry Res. (18), 197-201, 1986. [2]. Karson et al., Psychiatry Res. (45), 95-104, 1992. [3]. Renshaw et al., Am J Psychiatry (149), 1592-4, 1992. [4]. Komoroski et al., MRM (31), 204-11, 1994. [5]. Bartels et al., J Neural Transm Gen Sect. (99), 1-6, 1995. [6]. Strauss et al., Am J Psychiatry (154), 516-22, 1997. [7]. Henry et al., Am J Psychiatry (157), 1506-8, 2000. [8]. Bolo et al., Neuropsychopharmacology (23), 428-38, 2000. [9]. Strauss et al., Biol Psychiatry (49), 798-802, 2001. [10]. Henry et al., Neuropsychopharmacology (30), 1076-83, 2005. [11]. Henry et al., MRM (42), 636-42, 1999. [12]. Lange et al., JMRI (33), 748-54, 2011. [13]. Helms et al., MRM (46), 395-400, 2001. [14]. Ernst et al., MRM (65), 13-7, 2011.Figures