3882
The effects of iodinated CT contrast agent on phosphorus MRS1Oxford Centre for Clinical MR Research (OCMR), RDM Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom, 2Department of Imaging Methods, Institute of Measurement Science, Slovak Academy of Sceinces, Bratislava, Slovakia, 3Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom, 4The Wolfson Brain Imaging Centre, University of Cambridge, Cambridge, United Kingdom, 5Department of Physics, University of Oxford, Oxford, United Kingdom
Synopsis
Contrast-enhanced CT examination can influence 1H-MRI measurements performed within 24h after the CT scan, due to a reduction in water T1 and T2 caused by the iodinated contrast agents used in CT. We have investigated whether contrast from a previous CT examination would also influence metabolic measurements made using 31P-MRS, by measuring the T1 of 1H and 31P signals in human blood. We find that iodinated CT contrast agent has no effect on phosphorus T1s. Therefore, 31P-MRS examinations will not be influenced by prior CT (unlike 1H-MRI scans).
Introduction:
Cardiac MRI is a routine non-invasive imaging modality used to diagnose heart disease. It can quantify cardiac anatomy, function, perfusion and cardiac tissue energy metabolism. The latter is mainly probed by in vivo phosphorus MR spectroscopy (31P-MRS), which allows measurement of high-energy metabolites, e.g. adenosine triphosphate (ATP) and phosphocreatine (PCr). The PCr/ATP concentration ratio has been shown to change in most major cardiac disease states1,2. However, owing in part to long scan times and comparatively limited spatial resolution, it is common to combine MRI with other imaging modalities for a more complete characterisation of cardiac disease. In particular, contrast-enhanced computed tomography (CT) is frequently used. As it is beneficial to schedule the CT and MR examinations in close succession, it is important to identify any potential interference between the two examinations. CT contrast agents contain iodine and act to shorten the T1 and T2 relaxation times of water signals in proton (1H) MRI for up to 24 hours after the CT scan3,4. However, any potential confounding effects on 31P-MRS remain unknown.
Therefore, our aim was to determine whether an iodinated contrast agent affects the longitudinal (T1) relaxation time of 31P metabolites, and thus whether an earlier contrast enhanced CT scan would potentially influence the quantification of the cardiac PCr/ATP ratio.
Materials and Methods:
All measurements were performed using a vertical bore 11.7T MRI system (Bruker Avance) equipped with a dual-tuned 1H/31P RF 20mm diameter birdcage coil (Rapid Biomedical).
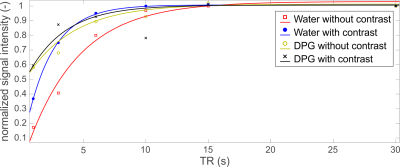
Fresh human blood (4 × 4mL vials) was obtained from healthy volunteers via antecubital fossa venepuncture and stored in EDTA buffer (Vacutainer, BD Healthcare). T1 relaxation times were measured on both channels (1H and 31P) in one vial sequentially after sampling using a non-localized pulse-acquire progressive saturation sequence with 6 TRs (1s, 3s, 6s, 10s, 15s and 30s), hard pulse excitation (150ms duration, θ≈60° nominal flip angle), 32 averages, sw=30kHz. The T1 measurement was performed before and repeated after the addition of a clinically indicated dose of iodinated contrast agent, Iopamidol (1.5mL/kg, i.e. 80mL/vial), and the whole experiment was finished within 2 hours after blood sampling. Water signal from the 1H spectra and 2,3-diphosphoglycerate (2,3-DPG) signal from the 31P spectra were fitted as Lorentzians using a Matlab (Mathworks) implementation of AMARES5, and used for determining mono-exponential T1 relaxation curves in Matlab. The two peaks of 2,3-DPG were summed together for the mono-exponential fitting of T1s.
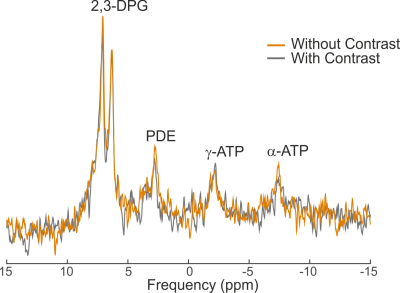
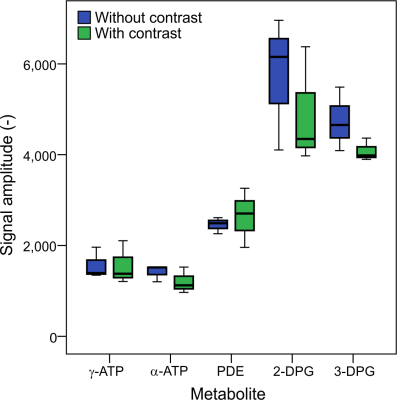
The remaining three vials were then used to acquire high SNR, partially saturated 31P spectra (TR=1s, θ≈60°, 512 averages) before and after the addition of Iopamidol. The same fitting routine was used to fit the signals of 2,3-DPG, phosphodiesters (PDE), γ-ATP and α-ATP. A paired unequal variance t-test was used to compare the fitted signals with and without contrast agent, with p<0.05 considered statistically significant.
Results and Discussion:
Figure 1 depicts the T1 fits of water and 2,3-DPG signals from 1H and 31P MRS acquisitions of blood, respectively. The water T1 shortened after adding Iopamidol from 4.8±1.9s to 2.2±0.1s. This is in good agreement with previous observations of reduced proton T1 in MRI after using iodinated contrast agents3,4. On the other hand, the T1 of DPG remained unchanged after mixing with the contrast agent, 3.7±1.2s to 3.0±0.3s, respectively. This could be expected as the 31P metabolites in blood are predominantly intracellular, and thus, there is no direct contact with the extracellular contrast agent that would influence the relaxation time, as is the case for the water signal. The effect on transverse relaxation (T2) of water4,6 was ignored in this study as 31P metabolites have short T2 times without contrast, and thus free induction decay signals are typically acquired in 31P-MRS7. Figure 2 depicts representative high SNR spectra of the blood acquired before and after adding Iopamidol, not showing any visible difference. Similarly, Figure 3 shows a box-plot diagram of fitted metabolite signals showing no difference. Table 1 summarizes the fit details in all three vials with and without contrast, with no significant differences found. This supports our finding of no change in the longitudinal relaxation time after adding the contrast agent (Figure 1), suggesting that metabolic examination using 31P-MRS after a contrast enhanced CT scan using the Iopamidol contrast agent would not be confounded.Conclusion:
This study demonstrates that CT contrast agent Iopamidol, while having T1 shortening effect on water signal, has no measurable effect on the 31P-MRS signal. Therefore, we propose that 31P-MRS datasets obtained in patients shortly after contrast-enhanced CT are directly quantifiable and comparable to those obtained without prior administration of CT contrast.Acknowledgements
We acknowledge financial support from the British Heart Foundation (FS/10/002/28078; FS/14/17/30634; RG/11/9/28921), a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (098436/Z/12/Z), a Novo Nordisk Foundation Postdoctoral Research Fellowship and from the Slovak Grant Agencies VEGA (2/0001/17) and APVV (15-0029).References
1. Bottomley P, et al. Radiology 1987;165:703-72. Neubauer S, et al. Circulation 1992;86:1810-8
3. Jinkins JR, et al. Am J Neuroradiol 1992;13:19-27
4. Hergan K, et al. Eur J Radiol 1995;21:11-7
5. Purvis LAB, et al. PloS ONE 2017;12:e0185356
6. Ogura A, et al. Acad Radiol 2009;16:1196-200
7. Valkovič L, et al. Anal Biochem 2017;529:193-215
Figures