3875
Can sodium triple-quantum signal separate extra- and intracellular signals? – investigation on HEP G2 liver cells, liposomes and nanoparticles1Computer Assisted Clinical Medicine, Heidelberg University, Mannheim, Germany, 2Institute of Functional Interfaces, Karlsruhe Institute of Technology, Karlsruhe, Germany
Synopsis
Sodium MRI is increasing in popularity albeit the apparent challenges of low SNR and fast bi-exponential decay. Currently, intra- and extra cellular sodium can only be resolved by introducing a chemical shift reagent which is unusable in human studies due to toxicity. There is discussion on whether triple-quantum signal (TQS) could provide a discriminator for resolving intra- and extracellular signal due to motion restriction of sodium ions within the cell. This work investigates the TQS behavior using a triple quantum sequence with time proportional phase increment (TQTPPI) on liposomes, HEP G2 liver cells and nanoparticles to disentangle the reasons for occurring TQS.
Introduction:
Sodium MR techniques are anticipated to provide valuable information about cell physiology and viability and could be useful as a biomarker in-vivo.1,2,3 Multi-quantum coherences are investigated as it is expected that based on the triple-quantum signal (TQS) extra- and intracellular signals can be distinguished.4,5 Currently, extra- and intracellular sodium can only be resolved by introducing a chemical shift reagent which is unusable in human studies due to toxicity.6 From literature7,8 the occurrence of the TQS depends on the limitation of sodium-ion movement. A persisting task, however, is the better understanding of 23Na TQS in the closed compartments of cells. Liposomes have been used previously to model biological membranes8 and can function as cell-phantom to study limited freedom of 23Na-ion movement due to partial trapping.10,11,12 PLGA nanoparticles have broad applicability in biomedical science and have been chosen as a closed compartment model of cell size without the characteristic double lipid membrane of cells.13 This work investigates the TQS behavior in HEP G2 liver cells, liposomes, and nanoparticles, to find out if TQS can be a discriminator for extra- versus intracellular sodium signal.Methods:
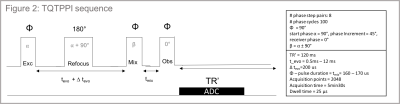
Data acquisition: Data were acquired on a 9.4T small-animal scanner (Biospec, Bruker, Germany) using an in-house built transmit/ receive 23Na surface coil. The sequence uses triple quantum time proportional phase increments (TQTPPI)5 (figure2) to study the 23Na – NMR spectrum, detecting simultaneously the single-quantum (SQ) and triple-quantum coherences (TQ) while suppressing double-quantum coherences. We qualitatively evaluated the TQS for each resulting spectrum by normalizing the TQ peak height to its respective single-quantum signal (SQS).
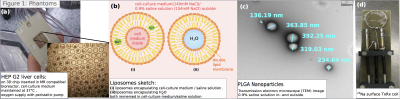
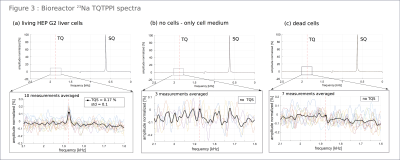
Phantoms: We studied HEP G2 liver cells in an MR compatible bioreactor (figure1A) filled with (a) living cells (b) cell-culture-medium only (c) and dead cells. Next, we investigated the TQS in liposomes in four different mixtures (figure1b,c). The first liposomal dispersion was prepared using 14ml of cell-culture medium (ThermoFisherScientific), 310µl safflower-oil, 202µl glycerin and 1.5µl vitamin-E. The components were mixed for 10min at 5000rpm; afterward, sonication was used for 30x1min to produce stable liposomes. Secondly, we produced liposomes with 0.9% saline solution instead of cell-medium and thirdly, liposomes encapsulating water dispersed in the saline solution. The fourth liposome mixture was prepared from soy-lecithin and saline solution, again following the mixing and sonication steps. Mean liposome size was measured by fixed angle dynamic light scattering to be 309nm. Soy lecithin liposomes were much smaller in size with 81nm. PLGA nanoparticles encapsulating saline-solution were prepared following the recipe from McCall et al.14 For reference we measured the signal in the nanoparticle phantom (figure1d) as well as in saline solution and a cell-medium-glycerin mixture. Nanoparticle diameter was measured by transmission-electron-microscopy(TEM) to be in the order of 130-400nm(figure1d). A volume of 10-15ml solution was measured for all phantoms.
Results:
The resulting spectra obtained by the TQTPPI-sequence are grouped in bioreactor (figure3), liposome (figure4) and reference-results(figure5). Figure3 showed a pronounced TQS for living cells, whereas for dead as for no cells, we obtained no TQS. Figure4 depicts a noticeable TQS for the liposome-phantoms(a,b,c). There was no visible difference in TQS using saline solution or cell-medium. Despite the smaller diameter of soy-lecithin liposomes, TQS result was similar. In contrast, liposomes filled with H2O (d) showed no TQS. Nanoparticle phantom TQS was low compared to background noise (figure5a). For reference, we measured TQS in pure saline-solution (figure5b), which also showed a weak TQS signal, against background noise. Cell-culture-medium–glycerin mixture (figure5c) showed no TQS.Discussion:
We found a pronounced TQS in living cells, as expected. Due to the absent peak in dead cells, TQS formation owing a differing viscosity inside the cell with present cell-organelles can be excluded. When producing liposomes directly with a sodium-ion solution, these ions can be trapped within the double lipid membrane15,16, then TQS is possibly due to this trap and not the intracellular-space limitation. This assumption was supported by the measurements of the liposome-water dispersion where no ions were trapped within the double lipid membrane as well as the nanoparticle measurements, where equal space limitation without double-lipid membrane does not produce a distinct TQS.Conclusion:
The combination of measurements suggests that the confinement of motion within a cell-sized compartment is insufficient to produce a TQS. However, sodium-ions trapped inside the double lipid membrane produces a TQS. In case of living cells, TQS then occurs only due to the binding to proteins, such as the sodium-potassium pump. Therefore, based on our findings, TQS does not solely originate from intracellular space limitation. However, it can be used as a biomarker for cell viability as it reflects the activity in transmembrane transport. It would be interesting to quantify the TQS for different disease states to find an onset value indicating ion pump failure.Acknowledgements
We thank the Electronmicroscopy Core Facility (EMCF), Heidelberg for providing the TEM images of the nanoparticle phantom.References
1. Bottomley, PA 2016, 'Sodium MRI in human heart: A review' NMR in Biomedicine, vol 29, no. 2, pp. 187-196.
2. S.P. Yu, L.M. Canzoniero, D.W. Choi, Ion homeostasis and apoptosis, Curr. Opin. Cell Biol. 13 (4) (2001) 405–411.
3. C.D. Bortner, J.A. Cidlowski, Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death, J. Biol. Chem. 278 (40) (2003) 39176–39184.
4. Schepkin, V. D., Neubauer, A., Nagel, A. M., & Budinger, T. F. Comparison of potassium and sodium binding in vivo and in agarose samples using TQTPPI pulse sequence. Journal of Magnetic Resonance, 277, (2017) 162–168.
5. Neubauer, A., Nies, C., Schepkin, V. D., Hu, R., Malzacher, M., Chacón-Caldera, J., Schad, L. R. Tracking protein function with sodium multi quantum spectroscopy in a 3D-tissue culture based on microcavity arrays. Scientific Reports, 7(1), (2017) 3943.
6. Constantinides CD, Rogers J, Herzka D, Bolar D, Boada FE, Kraitchman DL, Bottomley PA. Superparamagnetic iron oxide MION as a potential contrast agent for 23Na MRI in myocardial infarction. Magn. Reson. Med. (2001); 46: 1164–1168.
7. J Pekar, J S Leigh, Detection of biexponential relaxation in sodium-23 facilitated by double-quantum filtering, In Journal of Magnetic Resonance (1969), Vol. 69, Issue 3, 1986, 582-584
8. S. Hubbard, Paul. Non Exponential Nuclear Magnetic Relaxation by Quadrupolar Interaction. The Journal of Chemical Physics. (1970).
9. Perkins W R, Minchey S R, Ahl P L and Janoff A S The determination of liposome captured volume Chem. Phys. Lipids 64 (1993) 197–217
10. S.M. Johnson and A.D. Model Membranes. Bangham Biochim. Biophys. Acta 193, (1969) 82-91.
11. H. Hauser, M.C. Phillips and M. Stubbs. Ion Permeability of Phospholipid Bilayers. Nature 239, (1972) 342-344.
12. A. Carruthers and D.L. Melchior. How bilayer lipids affect membrane protein activity. Biochemistry 22, (1983) 5707-5807.
13. F Danhier, E Ansorena, J M. Silva, R Coco, A Le Breton, V Préat, PLGA-based nanoparticles: An overview of biomedical applications, In Journal of Controlled Release, Volume 161, Issue 2, (2012) 505-52.
14. McCall, R. L., & Sirianni, R. W. PLGA Nanoparticles Formed by Single- or Double-emulsion with Vitamin E-TPGS. Journal of Visualized Experiments : JoVE, (82), (2013)
15. E London, Lipid Bilayer Structure, In Encyclopedia of Biological Chemistry, edited by William J. Lennarz and M. Daniel Lane, Elsevier, New York, (2004), Pages 576-579.
16. D W. Deamer, J Bramhall, Permeability of lipid bilayers to water and ionic solutes, In Chemistry and Physics of Lipids, Volume 40, Issues 2–4, (1986), Pages 167-188
Figures