3871
Investigating the 23Na Flip-Angle Effect in Cartilage, Skin, and Muscle1Biomedical Engineering, University of Alberta, Edmonton, Canada, 2University of Victoria, Victoria, Canada, 3Biomedical Engineering Department, University of Alberta, Edmonton, Canada
Synopsis
The measurement of sodium concentration in cartilage, skin, and muscle with 23Na MRI requires the knowledge and minimization of spin 3/2-related signal losses. Here, a common B1 mapping experiment is used here to show that greater than prescribed flip-angles are produced in healthy human cartilage, skin, and muscle and that this effect is increased with longer RF excitation pulses. This points to the presence of residual quadrupole interactions in these tissues. To avoid the concomitant signal loss associated with residual quadrupole interactions, very hard (short) RF excitation pulses may be required.
Introduction
Spin 3/2 nuclei like 23Na possess electric quadrupole moments that align with environmental electric field gradients in tissue, and their long-lived orientation with respect to Bo will split the resonance into three frequencies. In the presence of these residual quadrupole interactions (RQI – with splitting frequency ωQ), the central resonance will flip at a rate greater than ω1 (ω1=γB1) when ω1 < ωQ (producing a greater flip-angle than that prescribed) [1,2]. The concern for MRI-based 23Na concentration measurement is that the detection of an increased flip-rate implies concomitant irretrievable signal loss [1,2]. 23Na RQIs have previously been detected in cartilage [3,4], but the presence of an increased rate of flipping (or a flip-angle effect) has not yet been evaluated in either cartilage, skin or muscle.Methods
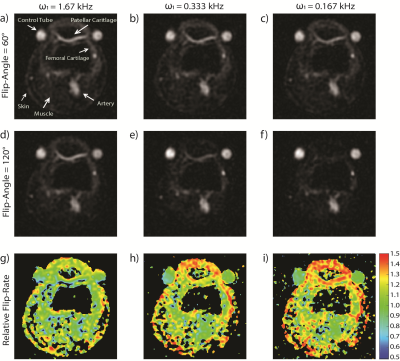
All sodium MRI experiments were performed on the right knees of 5 healthy volunteers (3 male, 2 female, age 19-40) using a 4.7T Varian Inova and a birdcage RF coil. For each volunteer, two control tubes filled with 4% agar and 112 mM [Na+] were placed on either side of the patella. A common B1 mapping experiment was used to investigate tissue dependent differences in rate of central resonance flipping. This experiment consisted of the acquisition of two images, one with a prescribed 60° flip-angle and the other 120° (based on tissue-average power calibration). From this experiment relative flip-rate can be calculated as described in [5] for brain. Each image was acquired with TR=180 ms to avoid a steady-state effect. Because the RQI flip-angle effect is manifest within the central resonance (which accounts for 40% of spin 3/2 signal and decays as a rate of T2slow), a relatively long TE of 2.05 ms was selected to avoid signal from the outer resonances which decay much more rapidly. This experiment was performed 3 times with ω1 values of 1.67 kHz, 0.333 kHz and 0.167 kHz (60° rectangular RF pulse lengths = 100/500/1000 µs, and 120° pulse lengths = 200/1000/2000 µs, respectively). Axial images were acquired using 2000 twisted projections with 18 ms readouts in 6 min with FOV=120 mm isotropic and voxel volume (defined by 1/2kmax) equal to 2.65×2.65×10.6 mm3. For each ω1 experiment a relative flip-rate map can be produced directly from the 60° and 120° images (as shown). However, for specific tissue assessment, average values within a 3D volume of interest (VOI) were measured from each (co-registered) image before non-linear flip-rate calculation. Tissues of interest included patellar and femoral knee cartilage, skin, muscle and the popliteal artery (which should not exhibit a flip-angle effect). A calculated relative flip-rate value of 1.0 implies that the prescribed 60° and 120° flip angles have been achieved. However, imperfect power calibration will globally scale relative flip-rate throughout the knee. For this reason the relative flip-rates calculated in each tissue are given with respect to the average value calculated from the two control tubes. In the absence of B1 variation (as will be discussed) a normalized relative flip-rate value of 1.0 implies no flip-angle effect. A paired t-test was used to test for significant difference (p<0.05) in flip-angle effect between the different ω1 experiments.Results
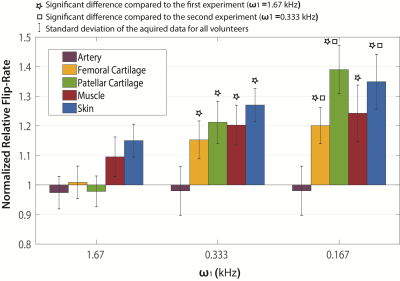
Representative slices from each of the six images acquired from one volunteer are shown in Figure 1 (a-c, flip-angle=60°; d-f, flip-angle=120°), along with relative flip-rate maps (g-i). Greater signal intensity on the 60° images compared to 120° is reflected in an increased flip-rate. This is particularly apparent for patellar cartilage on the ω1=0.167 kHz experiment images. Normalized relative flip-rate is plotted in Figure 2. As expected, the flip-rate in the artery is the same as the phantoms and is not altered with ω1. When very hard RF pulses are used (ω1=1.67 kHz) the relative flip-rate of cartilage is unchanged from the phantoms; however, that is not the case for muscle and skin. For cartilage and skin the relative flip-rate is significantly increased with each ω1 reduction; for muscle the flip-rate is only significantly increased between ω1=1.67 kHz and ω1=0.333 kHz.Discussion and Conclusion
A clear 23Na flip-angle effect is demonstrated in cartilage, muscle and skin. Local tissue-specific changes, the absence of an effect in the popliteal artery, and ω1 dependence suggest that the results measured are not simply those of coil-dependent spatial B1 variation. A flip-rate increase was not measured in cartilage when ω1=1.67 kHz (90° RF pulse length=150 μs) suggesting minimal signal loss due to residual quadrupole interactions. However, for muscle and skin even harder RF pulses may be required to avoid concomitant signal losses.Acknowledgements
Funding from the Natural Sciences and Engineering Research Council of Canada.References
[1] Pandey L, Towta S, Hughes DG. NMR pulse response and measurement of the quadrupole coupling constant of I =3/2 nuclei. J Chem Phys 1986;85:6923–7.
[2] Joseph PM, Summers RM. The flip‐angle effect: A method for detection of sodium‐23 quadrupole splitting in tissue. Magn Reson Med 1987;4:67–77.
[3] Reddy R, Insko EK, Noyszewski EA, Dandora R, Kneeland JB, Leigh JS. Sodium MRI of human articular cartilage in vivo. Magn Reson Med 1998;39:697–701.
[4] Ling W, Regatte RR, Schweitzer ME, Jerschow A. Behavior of ordered sodium in enzymatically depleted cartilage tissue. Magn Reson Med 2006;56:1151–5.
[5] Stobbe RW, Beaulieu C. Residual quadrupole interaction in brain and its effect on quantitative sodium imaging. NMR Biomed 2016;29:119–28.
Figures