3868
Design of a Dual Saddle Coil Setup for High Resolution 1H/23Na MRI of Ex Vivo Rat Tail Intervertebral Discs at 14.1T1Biomedical Engineering, The Pennsylvania State University, State College, PA, United States, 2Mechanical and Nuclear Engineering, The Pennsylvania State University, State College, PA, United States, 3Science Dept., Deltona High School, Deltona, FL, United States, 4State College High School, State College, PA, United States, 5Huck Institutes of the Life Sciences, The Pennsylvania State University, State College, PA, United States
Synopsis
Rat-tail intervertebral disc puncture is a commonly used model for studying treatment options and the pathology of Degenerative Disc Disease (DDD). However, longitudinal studies are impossible as analysis through biochemistry and histology requires euthanization. High-field MRI provides a possible solution to this problem as initial structural and compositional changes related to disc degeneration are observable. In this work, a dual saddle coil was constructed for 1H/23Na imaging at 14.1T. It was concluded from ex vivo rat-tail proton and sodium images that sodium MRI is feasible, and it could be an effective method for rat-tail intervertebral disc degeneration evaluation.
Introduction
Degenerative Disc Disease (DDD) is the gradual decay of the intervertebral discs that occurs with age or due to injury. Despite affecting millions of people, the onset and progression of disc degeneration are not well understood. One of the initial signs of DDD is a decrease in glycosaminoglycan content, which translates into a reduction in sodium concentration in the disc. Therefore, sodium MRI can potentially detect changes in composition before structural changes occur [1]. Current analysis methods, such as histology assessments and biochemistry, require invasive procedures. High-field magnetic resonance imaging (MRI) may provide a better alternative for DDD evaluation [2]. 23Na MRI paired with high resolution proton imaging can potentially measure structural and compositional disc changes in vivo for longitudinal studies. Puncture of the intervertebral discs of rat tails is a commonly used model of human DDD [3,4]. The objective of this study is to design a dual saddle coil radio frequency (RF) transmit/receive probe-head for sodium and proton imaging of ex vivo rat tails at 14.1T.Methods
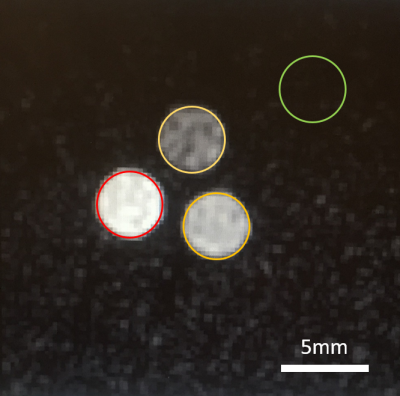
Imaging was conducted on a 14.1T micro imaging system. Two housed saddle coils were constructed resonating at 600MHz (1H) and 159MHz (23Na) respectively. The inner sodium resonator had a diameter of 14mm, the outer proton resonator had a diameter of 20mm. The height of both coils was 30mm to capture two to three intervertebral discs within the homogenous area of the coils. The coils were fabricated from 22 gauge copper wire. The structural components of the probe-head were designed in Solidworks and 3D printed using PLA. Bazooka baluns were integrated into each transmission line. Standard coupling networks were attached to both channels. Additional fixed capacitors needed to be added to the circuits to operate at the Larmor frequencies (see Figure 1). An 8μm copper foil shield mounted on garolite covered the coils to prevent gradient coil interferences. Initial testing of the setup was done using standard gradient echo sequences with three phantoms containing 100mM, 200mM, and 300mM NaCl solutions respectively (see Figure 2). Regarding sodium imaging, the echo time was minimized to achieve maximum signal. A rat tail harvested from a transcardial perfusion fixed animal was inserted into the resonator and its intervertebral discs were imaged using both sodium and proton MRI.Results
The different sodium concentrations could clearly be distinguished in the phantom experiment (see Figure 2; proton data not shown).
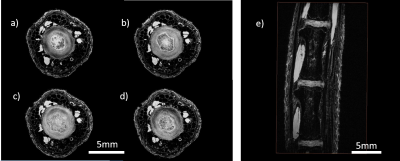
The resolution of the ex vivo rat tail proton data set was 50μm isotropic (TE: 5.61ms, TR: 40.00ms, FOV: 15 x 15 x 25mm3 ; matrix: 300 x 300 x 500). With 16 averages, the total scan time was 16hrs. Data reconstruction was conducted in Matlab (The Mathworks Inc., Natick, MA). After zero filling by a factor of two, a 25μm isotropic pixel resolution was achieved. Besides connective tissue, skin layers, bone, and muscle; the nucleus pulposus (NP) and the annulus fibrosus (AF) could be imaged with very high signal to noise ratios (SNR) (see Figure 3).
Sodium images were acquired using the following parameters: TE = 1.25ms, TR = 40.00ms, 403mm3 FOV, and a 48 x 48 x 48 matrix size resulting in an 833μm isotropic resolution. After zero filling by a factor of four, a 208μm isotropic pixel resolution was achieved. The scan comprised 128 averages and 3hrs and 17min scan time. The discs, muscle, and skin layers could be identified. The NP showed a higher SNR than the AF as expected (see Figure 4).
Discussion
This study demonstrates the feasibility of sodium imaging with an adequate resolution for analyzing rat-tail intervertebral discs. It was determined from the phantom images that there was enough contrast between the different phantoms to be able to observe disc degeneration over time. While the sodium images provided mainly compositional information, structural changes caused by disc degeneration could be analyzed using the high resolution proton images. Due to the high SNR, a dramatic reduction in scan time seems viable to allow for in vivo experiments. Colocalization of sodium and proton images can provide valuable information on the relationships between structure and composition in the pathological intervertebral disc.Conclusion
This study showed the hardware development and feasibility data of using high-field sodium and proton MRI as a method of analyzing composition and structure in a rat model of DDD. Comparing structural and compositional changes in the discs over time could provide new information about the onset of DDD and its progression. Future studies will focus on analyzing temporal changes in the process of disc degeneration using the technological developments presented in this study.Acknowledgements
Preliminary imaging was supported by the Huck Institutes of the Life Sciences endowment fund at The Pennsylvania State University, PA, USA .References
[1] C. Wang, E. McArdle, W. Witschey, M. Elliott, R. Reddy, and A. Borthakur, “Validation of Sodium MRI of Intervertebral Disc Degeneration,” Proc. 17th Sci. Meet. Int. Soc. Magn. Reson. Med., vol. 35, no. 5, p. 294, 2009.
[2] N. Inoue and A. Espinoza Orias, “Biomechanics of Intervertebral Disc Degeneration,” Orthop Clin North Am, vol. 42, no. 4, pp. 487–499, 2011.
[3] C. Daly, P. Ghosh, G. Jenkin, D. Oehme, and T. Goldschlager, “A Review of Animal Models of Intervertebral Disc Degeneration: Pathophysiology, Regeneration, and Translation to the Clinic,” Biomed Res. Int., vol. 2016, 2016.
[4] B. Han et al., “A Simple Disc Degeneration Model Induced by Percutaneous Needle Puncture in the Rat Tail,” Spine (Phila. Pa. 1976)., vol. 33, no. 18, pp. 1925–1934, 2008.
Figures