3867
Sodium imaging with an UTE technique to study the effects of exercise on muscle.1Sir Peter Mansfield Magnetic Imaging Centre, University of Nottingham, Nottingham, United Kingdom
Synopsis
Sodium (23Na) imaging can provide insight into the effects of exercise on muscle. Here we perform a 3D dual TE UTE scheme with radial FID readout to assess the alterations in sodium tissue concentration in the gastrocnemius and soleus muscles; immediately after exercise performed inside the scanner using a Trispect pedal system. In addition an mDIXON proton scan was acquired for delineation of the muscle groups. Following exercise, the time course of the 23Na signal intensity in the medial gastrocnemius and soleus muscles was found to return to baseline after approximately 30 minutes, with a 10 – 20 % signal change.
INTRODUCTION
Sodium (23Na) imaging can provide insight into the effects of exercise on muscle1,2. Exercise causes a change in the exchange of metabolites and water between extracellular and intracellular spaces which is reflected by changes in the large 23Na gradient across the extracellular and intracellular space. Since 23Na undergoes quadrupolar interactions, its T2* signal decay is bi-exponential. In order to detect the effects of exercise in the muscle, fast T2*components (0.5-3 ms) must to be captured, This work uses a 23Na surface coil and ultrashort echo-time (UTE) imaging to capture the bi-exponential decay of 23Na. This is subsequently applied to measure changes in 23Na concentration in response to anaerobic exercise.METHODS
Scanning was performed on a 3T Philips Achieva scanner using a 13 cm diameter single channel 23Na surface RF coil for optimal imaging of the calf muscle. A 3D radial FID readout with dual TE using a non-selective RF block pulse for excitation was optimised for imaging the flexor muscles used in the exercise. Dual echo time data was collected at TE1/TE2 = 0.24/2.9 ms (10 axial slices, resolution 3x3x30 mm, FOV 192x192 mm, NSA = 30, acquisition time = 6:34 mins). This was compared to a FLASH acqusition3 (total acquisition time, TA = 13.7 minutes; echo time, TE = 2.07 ms; repetition time, TR = 100 ms; flip angle, FA = 90°; 128 averages, resolution: 3×3×30 mm3) and found to provide a factor of 3 improvement in SNR as well as improved spatial coverage. Two subjects (2M; 22-23yrs) then performed a high intensity anaerobic exercise protocol while supine on the scanner bed using a Trispect pedal system (Ergospect, GmbH, Austria). Subjects were instructed to exercise to fatigue. Immediately following this, 3D 23Na scans were serially collected every 6:34 minutes to image recovery following exercise. Following these 23Na scans, a high resolution mDIXON proton scan was acquired in the same space to allow for accurate muscle region segmentation (50 axial slices, resolution 1x1x6 mm, FOV 192x192 mm, TA = 2:16 mins). The total scan duration was 53 minutes.
Data analysis: All analysis was performed in Matlab. 23Na images were corrected for the B1 field inhomogeneity. The mDIXON proton images were used to manually segment the gastrocnemius and soleus muscles (Fig.1). These regions of interest were then mapped onto the 23Na images which were interrogated. The percentage change in the average 23Na signal intensity in both the gastrocnemius and soleus muscle was then serially assessed from the first 23Na scan following exercise.
RESULTS
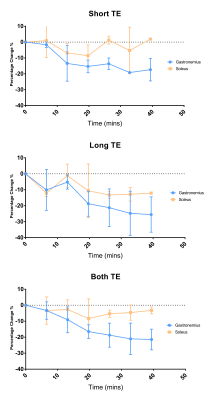
Figure 1 shows an example 23Na image at the short and long echo time, and the combination of the echo times. Figure 2 shows the time course of the 23Na signal intensity in the medial gastrocnemius and soleus muscles. At short TE, measurements shows a reduction in the sodium signal in medial gastrocnemius muscles by -19 ± 5 % (P = 0.007). At long TE, measurements show a reduction in the sodium signal in both the medial gastrocnemius muscle by -25 ± 8 % (P = 0.007) and in the soleus muscle by -13 ± 6 % (P = 0.006). Combining both short and long echo times a reduction in sodium signal was seen in the medial gastrocnemius muscle of -21 ± 6 % (P = 0.008) and in the soleus muscle by -5 ± 6 % (P = 0.007), with a return to baseline at approximately 30 minutes.DISCUSSION
These results show that it is possible to monitor the return of this 23Na signal to baseline following exercise in both the medial gastrocnemius muscle and the soleus muscles at 3T. The magnitude and half-life of change is in agreement with previous studies4,5. The reduced variance in the 23Na signal intensity measures at short TE compared to the long TE highlights the benefits of using a UTE sequence to capture the fast components of the bi-exponentially decaying T2* signal. It has been suggested that this observed change in sodium concentration arises as result of either intracellular Na+ accumulation caused by depletion of the Na+/K+-ATPase of activated muscle cells or increased perfusion of the activated muscles after exercise. Future studies will aim to assess these mechanisms.CONCLUSION
This study has demonstrated the feasibility of using a dual TE 3D radial FID sequence to monitoring physiological changes in the muscle immediately post-exercise. This has demonstrated the sensitivity of 23Na. In future this methodology will be applied to assess alterations in muscle physiology in patients, for example in myotonic dystrophy1.Acknowledgements
This work was supported by the Sir Peter Mansfield Imaging Centre, University of Nottingham and Philips Healthcare clinical systems and was funded by the MRC Discovery Award.References
- Constantinides CD, Gillen JS, Boada FE, Pomper MG, Bottomley PA. Human skeletal muscle: sodium MR imaging and quantification—potential applications in disease and exercise. Radiology 2000; 216:559–568.
- Bansal N, Szczepaniak L, Ternullo D, Fleckenstein JL, Malloy CR. Effect of exercise on 23Na MRI and relaxation characteristics of human calf muscle. J Magn Reson Imaging. 2000; 11:532–538.
- Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, Titze J.23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013 Mar;61(3):635-40.
- Hammon M, Grossmann S, Linz P, Kopp C, Dahlmann A, Janka R, Cavallaro A, Uder M2, Titze. 3 Tesla (23)Na magnetic resonance imaging during aerobic and anaerobic exercise. J.Acad Radiol. 2015 Sep;22(9):1181-90.
- Chang G, Wang L, Schweitzer ME, et al. 3D 23Na MRI of human skeletal muscle at 7 Tesla: initial experience. Eur Radiol 2010; 20(8):2039–2046.
Figures