3850
Analysis Methods for Human Hyperpolarized 13C-pyruvate Studies1Radiology and Biomedical Imaging, University of California - San Francisco, San Francisco, CA, United States, 2Electrical Engineering and Computer Science, University of California - Berkeley, Berkeley, CA, United States, 3Medicine, University of California - San Francisco, San Francisco, CA, United States, 4Pharmacy, University of California - San Francisco, San Francisco, CA, United States
Synopsis
A major challenge for human Hyperpolarized 13C metabolic MRI is to develop informative, accurate and robust methods for measuring metabolic conversion, while accounting for a broad range of experimental characteristics and without gold-standard experiments for evaluating accuracy and precision. We present a simulation framework to evaluate analysis strategies and show that an “input-less” kPL fitting method is a promising approach for accurate and robust measurements of metabolism in human hyperpolarized 13C-pyruvate MRI. We evaluate this method in human prostate cancer studies, where we observed variability of ±5-10s in the bolus delivery that can lead to errors in other analysis methods.
Introduction
Hyperpolarized 13C-pyruvate MRI is now entering more widespread clinical trials for studies of metabolism in cancer as well as heart disease. The interpretation of this study data requires informative, accurate and robust methods for measuring metabolic conversion tailored to human studies, which have shown a much broader range of bolus characteristics and perfusion compared to preclinical studies. Furthermore, multi-site studies will soon be conducted to evaluate this modality more broadly.
A major challenge in developing analysis methods is there is no gold-standard for evaluating accuracy, while analyzing precision requires an impossibly large number of studies. To address this, we propose a Monte Carlo simulation framework in which to evaluate to performance of analysis methods in response to expected experimental variability and unknown parameters. The results were evaluated in human hyperpolarized 13C-pyruvate MRI of primary prostate cancer.
Methods
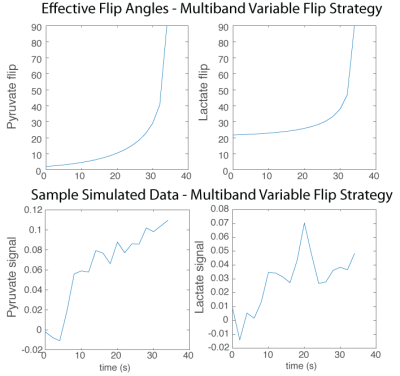
Data were acquired with a 3D dynamic MRSI sequence covering the entire prostate using a blipped EPSI acquisition with a compressed sensing reconstruction1. Other 3D MRSI sequence parameters included 12x12x16 matrix size, TE = 4.0 ms, TR = 150 ms, 8 mm isotropic resolution, and 2 sec between timepoints. Multiband spectral-spatial RF excitation pulses were used, combined with a variable flip angle strategy in time2.
Analysis methods used assumed uni-directional conversion from pyruvate to lactate with a rate constant kPL (kLP = 0), and metabolite decay rates R1L and R1P. All methods were modified to allow for arbitrary flip angle schemes, and are available in the hyperpolarized-mri-toolbox: https://github.com/LarsonLab/hyperpolarized-mri-toolbox
Area Under Curve ratio (AUCratio)3: For this method the ratio of the area under the lactate to area under pyruvate curves is used as a simple surrogate for metabolic conversion. A calibrated AUCratio was computed based on the nominal expected experimental parameters.
Boxcar-input kPL fitting: This fitting approach assumes a box-car input shape, including start time of the bolus, bolus duration, and the injection rate to characterize the input4.
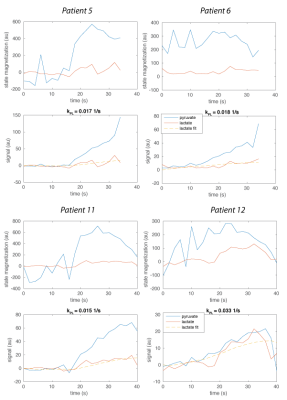
Input-less kPL fitting: In this approach, we only fit the lactate magnetization, not the pyruvate magnetization, where the measured pyruvate magnetization is used as the input for the kinetic model at each time point5. This model requires fitting just kPL and the metabolite decay rates, R1L and R1P.
Simulated data was generated based the two-site model, with a gamma-variate input function. Monte Carlo simulations were performed by adding random noise to evaluate the precision and accuracy of the fitting methods. Ranges of simulated values were chosen based on what we observed or estimated in human prostate cancer studies.
Results & Discussion
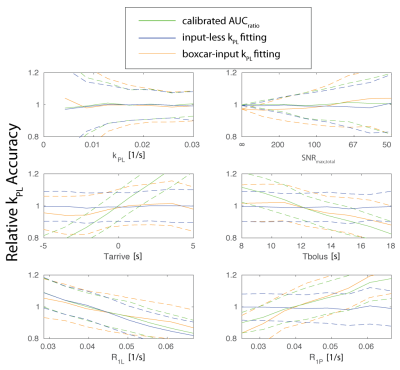
The Monte Carlo simulation results with approximately equivalent SNR to human prostate experiments show the input-less fitting outperforms both the AUCratio and boxcar-input fitting methods, with typical expected errors < 10%.
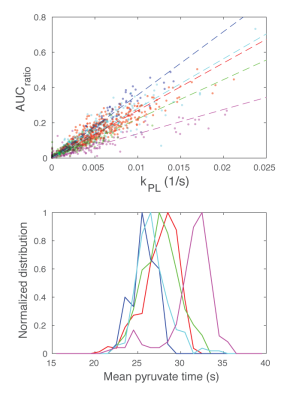
The variable flip strategy causes the AUCratio approach to have systematic biases when there is variation in the bolus timing (Tarrive), bolus duration (Tbolus), and when the pyruvate decay rate, R1P, deviates from the assumed value. The boxcar-input is more robust to the variations in the bolus timing and duration, but has a bias with deviations in R1P. Meanwhile, the input-less method is robust to R1P deviations as well. The performance of the boxcar-input fitting may improve by using a measured input function6-8.
All methods have systematic bias when there are deviations in the lactate decay rate, R1L, which was fixed to an assumed value. R1L can additionally be fit with the kPL fitting methods, but this leads to substantial increases in the expected error of > 20 % (would not appear on range plotted). This error when fitting R1L is greater than the bias+error across the range of R1L from 15 to 35 s when R1L is fixed, suggesting it is not a favorable tradeoff to include R1L in the kinetic model fitting.
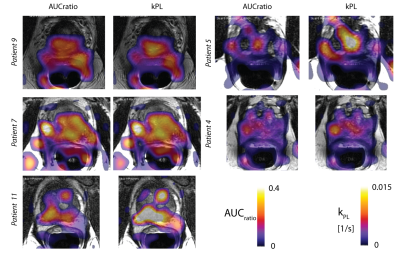
In human studies, we observed inter-subject variations in pyruvate delivery times to the prostate. AUCratio and input-less kPL fits show good agreement of spatial distributions but differ by apparent scaling factors, which can be explained by variations in bolus delivery. The mean pyruvate time, a measure of delivery time, shows ±5-10 s in arrival to the prostate. This variation, if unknown, is expected to lead to errors in the AUCratio, while the kPL fits should be unaffected.
Conclusion
An input-less kPL fitting method is a promising approach for accurate and robust measurements of metabolism in human hyperpolarized 13C-pyruvate MRI. This method is particularly advantageous for human studies, where we observed variability of ±5-10s in the bolus delivery that can lead to errors in the AUCratio method. The simulation framework provides a way to evaluate analysis strategies in response to potential experimental variations and unknown factors.Acknowledgements
This work was supported by grants from the NIH (R01EB017449, R01EB013427, R01CA166655, and P41EB013598).References
1. Larson PEZ, Hu S, Lustig M, Kerr AB, Nelson SJ, Kurhanewicz J, Pauly JM, Vigneron DB. Fast dynamic 3D MR spectroscopic imaging with compressed sensing and multiband excitation pulses for hyperpolarized 13C studies. Magn Reson Med. 2011 Mar;65(3):610–9. PMID: 20939089
2. Xing Y, Reed GD, Pauly JM, Kerr AB, Larson PEZ. Optimal variable flip angle schemes for dynamic acquisition of exchanging hyperpolarized substrates. J Magn Reson. 2013 Sep;234:75–81. PMID: 23845910
3. Hill DK, Orton MR, Mariotti E, Boult JKR, Panek R, Jafar M, Parkes HG, Jamin Y, Miniotis MF, Al-Saffar NMS, Beloueche-Babari M, Robinson SP, Leach MO, Chung Y-L, Eykyn TR. Model free approach to kinetic analysis of real-time hyperpolarized 13C magnetic resonance spectroscopy data. PLoS One. 2013;8(9):e71996. PMID: 24023724
4. Zierhut ML, Yen Y-F, Chen AP, Bok R, Albers MJ, Zhang V, Tropp J, Park I, Vigneron DB, Kurhanewicz J, Hurd RE, Nelson SJ. Kinetic modeling of hyperpolarized 13C1-pyruvate metabolism in normal rats and TRAMP mice. J Magn Reson. 2010 Jan;202(1):85–92. PMID: 19884027
5. Khegai O, Schulte RF, Janich MA, Menzel MI, Farrell E, Otto AM, Ardenkjaer-Larsen JH, Glaser SJ, Haase A, Schwaiger M, Wiesinger F. Apparent rate constant mapping using hyperpolarized [1-(13)C]pyruvate. NMR Biomed. 2014 Oct;27(10):1256–65. PMID: 25156807
6. Bankson JA, Walker CM, Ramirez MS, Stefan W, Fuentes D, Merritt ME, Lee J, Sandulache VC, Chen Y, Phan L, Chou P-C, Rao A, Yeung S-CJ, Lee M-H, Schellingerhout D, Conrad CA, Malloy C, Sherry AD, Lai SY, Hazle JD. Kinetic Modeling and Constrained Reconstruction of Hyperpolarized [1-13C]-Pyruvate Offers Improved Metabolic Imaging of Tumors. Cancer Res. 2015 Nov;75(22):4708–17. PMID: 26420214
7. Maidens J, Gordon JW, Arcak M, Larson PEZ. Optimizing flip angles for metabolic rate estimation in hyperpolarized carbon-13 MRI. IEEE Trans Med Imaging. 2016 May; PMID: 27249825
8. Sun C, Walker CM, Michel KA, Venkatesan AM, Lai SY, Bankson JA. Influence of parameter accuracy on pharmacokinetic analysis of hyperpolarized pyruvate. Magn Reson Med. 2017.
9. Larson PEZ, Kerr AB, Chen AP, Lustig MS, Zierhut ML, Hu S, Cunningham CH, Pauly JM, Kurhanewicz J, Vigneron DB. Multiband excitation pulses for hyperpolarized 13C dynamic chemical-shift imaging. J Magn Reson. 2008 Sep;194(1):121–7. PMID: 18619875
Figures