3841
SNR Requirements for Successful Application of Compressed Sensing Acceleration to Non-lipid suppressed 1H MRSI at Ultra-High Fields1Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2IMPRS for Cognitive and Systems Neuroscience, Eberhard-Karls University of Tuebingen, Tuebingen, Germany, 3Institute of Physics, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany
Synopsis
In this work we systematically investigate the requirements for successful application of compressed sensing for highly accelerating the acquisition of non-lipid suppressed 1H FID MRSI data at ultra-high fields. It is shown that with a combination of parallel imaging and sparse reconstruction, and an RF coil with an even distribution of receive sensitivity, highly accelerated and high resolution metabolite maps can be acquired at 9.4T through compressed sensing.
Introduction
The sparse nature of MR spectroscopy data has encouraged the use of compressed sensing (CS)1-2 to enable acceleration factors beyond those achieved by parallel imaging. While for the case of X-Nuclei the degree of sparsity is naturally sufficient to employ compressed sensing3-4, for 1H MRSI, due to the presence of multiple small and overlapping resonance peaks, applying CS is more challenging.
The problem is even more severe for the case of non-lipid suppressed ultra-short TR/TE MRSI due to the presence of strong lipid signals that prevent the CS reconstruction algorithm from recovering the metabolites of interest which have lower SNR in comparison.
In this work, we highlight the key factors leading to a reliable recovery of the signal despite the presence of strong lipid signals in non-lipid suppressed short TR FID MRSI at ultra-high fields, which include certain requirements on the RF coil and the necessity of combining parallel imaging and sparse reconstruction. Finally, we present high resolution in-vivo metabolite maps of the human brain acquired with CS acceleration at 9.4T.
Methods
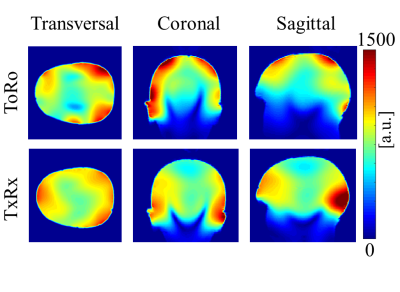
All experiments were conducted on a 9.4T Siemens whole-body human scanner. Two different in-house developed RF coils were used for comparison: a 16Tx/31Rx RF coil with transmit-only and receive-only coil elements5 (ToRo) with the receive elements arranged on a helmet shaped holder and an 18Tx/32Rx transceiver RF coil6 (TxRx) with 16 TxRx loops and 16 dedicated vertical receive loops arrange on a cylindrical holder. The ToRo coil has more receive sensitivity in the periphery and less in the center of the brain, but the TxRx coil has a more even distribution of receive sensitivity due to the way the vertical receive coils are optimized and due to the better coverage. Figure 1 shows a comparison of the SNR distribution of these coils on a head and shoulder phantom.
Fully sampled single-slice 1H MRSI data were acquired from healthy volunteers using a non-lipid suppressed ultra-short TR/TE 1H FID MRSI sequence7-9 with: FOV = 200mmx200 mm, slice thickness = 10mm, matrix size = 64x64, TE = 1.56ms, TR = 300ms, spectral bandwidth = 8kHz.
Four random variable-density undersampling masks were used leading to effective acceleration factors and scan times of R = 2, 4, 5 and 10, and TA = 7.5, 3.75, 3 and 1.5 minutes, respectively.
The accelerated data was reconstructed using compressed sensing optimization1 with a data consistency term, a total variation and a wavelet sparsity term. A coil-by-coil sparse reconstruction was compared to a combination of parallel imaging (using SENSE10) and sparse reconstruction where the coil channels are combined using when calculating the sparsity measures during the reconstruction to boost the SNR of the data.
Datasets from the two different RF coils were used to investigate the effect of the RF coils on CS reconstruction. After the coil comparison analysis, the best coil was used to compare different acceleration factors using CS at 9.4T.
Results/Discussion
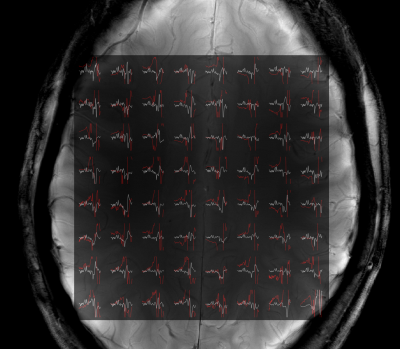
Figure 2 shows the results of the sparse versus SENSE-sparse reconstruction for R=4. The coil-by-coil sparse reconstruction (red) clearly has large lipid artifacts while the SENSE sparse reconstruction (white) is able to reliably recover the data. This is likely due to the fact that individual coil elements have lower SNR than the coil-combined data.
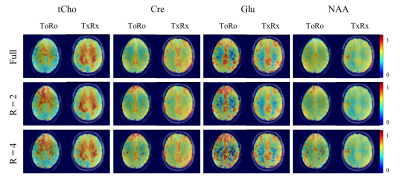
Metabolite maps acquired with the ToRo versus the TxRx coil are shown in Figure 3. The CS reconstruction performed better for the TxRx than for ToRo. Using the TxRx coil, all the metabolite maps are comparable to the fully sampled maps albeit slightly more noisy. On the contrary, the maps for the ToRo clearly degrade faster, especially in regions where the coil results in less SNR.
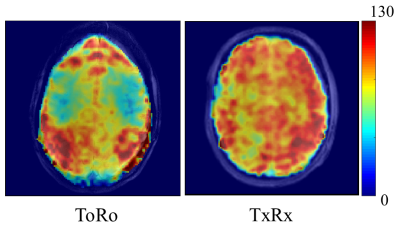
To further highlight the differences between the two coils, SNR maps calculated from the MRSI data are shown for each coil in Figure 4. The overall SNR for TxRx was higher and more evenly distributed than for ToRo.
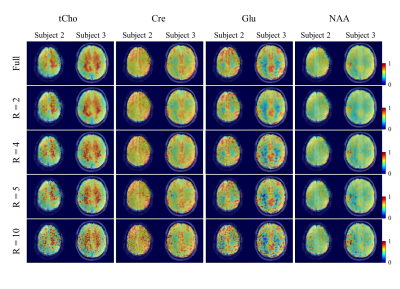
Finally, using the best CS reconstruction and coil, Figure 5 shows metabolite maps with increasingly higher acceleration factors. The maps show very good reconstruction for R = 2, and acceptable reconstruction for R = 4 and 5. Although quite noisy, the gray/white matter contrast can clearly be discerned for even high accelerations of R = 10.
Conclusion
In this work we showed that for a successful CS implementation the acceleration of metabolite maps at ultra-high fields, a combination of parallel imaging and sparse reconstruction must be used with an RF coil that has an even receive sensitivity distribution across the whole slice. Without these requirements the higher SNR of the subcutaneous lipid signals dominates the sparse reconstruction.Acknowledgements
No acknowledgement found.References
[1] Lustig et al, MRM 2007.
[2] Otazo et al, ISMRM 2009.
[3] Hu et al, MRM 2010.
[4] Askin et al, JMR 2010.
[5] Shajan et al, MRM 2014.
[6] Advievitch et al, NMR in Biomed 2017.
[7] Nassirpour et al, NeuroImage 2016.
[8] Henning et al, NMR in Biomed 2009.
[9] Bogner et al, NMR in Biomed 2012.
[10] Pruessman et al, MRM 1999.
Figures