3835
Towards an ultra-high field virtual biopsy for hepatic cancers using phospholipid metabolismDeb Rivera1,2,3, IML van Kalleveen3, Catalina Arteaga de Castro4, Hanneke Laarhoven3, Dennis Klomp1,4, Wybe van der Kemp4, Jaap Stoker3, and Aart Nederveen3
1MR Coils BV, Zaltbommel, Netherlands, 2Spinoza Centre, Amsterdam, Netherlands, 3Academic Medical Center, Amsterdam (AMC), Amsterdam, Netherlands, 4University Medical Center Utrecht (UMCU), Utrecht, Netherlands
Synopsis
At 7T 31P spectroscopy becomes possible on clinically relevant scales. Here we tackle the greatest challenge in multi-parametric imaging at high-field – creating localizers and radiological-grade images in the body in the absence of a bore body coil. By using antennas rather than loop coils, we image the full extent of the liver (n=10). Combining antennas with parallel transmit, allows radiological-grade images. Although technically feasible, it is not yet possible to obtain multi-nuclear spectra and parallel transmit images within the same scan session. We combine the methods by rebooting the scanner mid-exam, and demonstrate the method can be used for imaging patients.
Introduction
Phosphorus spectroscopy is a marker for treatment response in hepatic cancers,1,2 and at 7 Tesla clinically-relevant spatial resolutions (<18mL) and time scales (<30min) become feasible, allowing differentiation of relevant metabolites and clinical treatment-response tracking.3 There is no body coil in the bore of the 7T, nor receive loops in the bed. At 7T (~300MHz) collecting a radiological-grade anatomical image of the liver presents a challenge due to poor penetration depth4 and destructive interference.5 Although incapable of imaging even half of the liver at 300MHz, surface coils are the state-of-the-art for acquiring anatomical (1H) images for phosphorous spectroscopy, High field 31P spectroscopy in the liver has been limited to diffuse disease.6,7 Antennas enable improved penetration depths at 300MHz,8 and when combined with parallel transmit enable body imaging at 7T (Fig.1). Although technologically feasible, combination of multinuclear spectroscopy (non-proton) and parallel transmit is not yet available on commercial 7T scanners. This results in general in poor coverage of the liver. Here we present the use of antennas to improve penetration depth for multi-nuclear spectroscopy, as an improvement over loops for obtaining localizers, and demonstrate the need for the combination of parallel transmit with phosphorus spectroscopy for ultra-high field multi-parametric imaging of hepatic cancers.Methods
A custom-made liver coil (MR Coils BV, Zaltbommel) consisting of 8 30-cm dipole antennas tuned to the proton (298MHz) frequency, and 2 loops (20cm diameter) tuned for 31P (120MHz) were used to transmit and receive signal. The pairs of elements were combined with a fixed phase of 90-degrees (quad-hybrid). For power optimization for 31P, we swept the flip-angle to identify the maximum signal intensity in a phantom and adapted for a volunteer (maxB1=30μT). Adiabatic pulses were used (adiabatic half-passage with a tanh/tan shape, 2ms pulse duration, and maximum frequency modulation of 5kHz), thus we did not further optimize the transmit power for the multi-nuclear experiment. Similarly, the optimal power level was found and written into the coil file for the proton antennas (maxB1=10μT). Spectra voxels were selected from 3DiCSI and Hamming filtered, uploaded into JMRUI and filtered with a 40Hz Lorentzian, zero filled to double the number of points, zero-order phase corrected to maximize the closest non-split peak to the phospholipid metabolites, and 1st order (0.4ms) phase corrected.Results
Penetration depth of a single antenna exceeds 20 cm, as shown in a large phantom (Fig.2). Two antennas with fixed-phase allow the liver to be visualized in vivo (Fig.3). Aggregate data compare penetration depth (n=9) with liver depth (n=5) for each direction, with A/P penetration approximately equal to liver depth (Fig.3). The liver and full axial slice of the abdomen can be visualized with 8-transmit/receive antennas (Fig.1). Parallel transmit with 8-antennas allows for B1-shimming to overcome black-band image voids (Fig.4). Fig.5 shows results from a patient with 31P MRS projected onto a DIXON image (same scan parameters as Fig.1).Discussion and conclusions
We have developed a protocol for imaging hepatic cancer patients using parallel transmit and 31P spectroscopy sequentially at 7T. The field of view afforded by two antennas is sufficient to image the boundaries of the liver for CSI planning and region-of-interest-based B0 shimming for the 31P experiment. With such a setup it is possible to locate tumors anywhere within the liver in order to optimally position the imaging matrix of 3D CSI. Additionally, the 2-antennas allow for alignment with anatomical images collected using parallel transmit. Once 31P data is acquired using the standard (dual-channel/multi-nuclear) mode of the scanner, the system must be rebooted and changed to parallel-transmit mode for anatomical imaging using B1+ shimming. To our knowledge, 7T 31P studies have been limited to diffuse liver disease using loops for 1H imaging with partial coverage of the liver (<50%) and penetration depths of 10cm.6,7 We have demonstrated that with antennas we can achieve nearly full coverage of the liver, facilitating tumor localization and region-of-interest B0 shimming. The 31P protocol can be improved by B1 calibration, improved penetration depth, T1-saturation correction (or longer TR), use of a BIR4 adiabatic pulse for uniform phase across the bandwidth, and acquisition methods to improve SNR (e.g.9,6). Hardware improvements include the use of a 31P birdcage and an array of 31P receive coils.10 With the commercially available 7T platforms, it is not possible to collect multinuclear (non-proton) data and anatomical images using parallel transmit without rebooting the system. The implications of adapting treatments to patients with a virtual-biopsy for hepatic cancers using full-radiological grade images and multi-parametric imaging (31P, DWI), warrants adapting the commercially available 7T scanner systems to meet the clinical needs.Acknowledgements
Thank you to the Spinoza Centre, Michel Italiaander, and the engineers and staff at MR Coils.References
- Dixon RM. NMR studies of phospholipid metabolism in hepatic lymphoma. NMR Biomed. 1998 Nov;11(7):370-9.
- Meyerhoff DJ, Karczmar GS, Valone F, Venook A, Matson GB, Weiner MW. Hepatic cancers and their response to chemoembolization therapy. Quantitative image-guided 31P magnetic resonance spectroscopy. Invest Radiol. 1992 Jun;27(6):456-64.
- Glunde K, Jiang L, Moestue SA, Gribbestad IS. MRS and MRSI guidance in molecular medicine: targeting and monitoring of choline and glucose metabolism in cancer. NMR Biomed. 2011 Jul;24(6):673-90.
- Pang Y, Wu B, Wang C, Vigneron DB, Zhang X. Numerical Analysis of Human Sample Effect on RF Penetration and Liver MR Imaging at Ultrahigh Field. Concepts Magn Reson Part B Magn Reson Eng. 2011 Oct;39B(4):206-216.
- Van de Moortele PF, Akgun C, Adriany G, Moeller S, Ritter J, Collins CM, Smith MB, Vaughan JT, Uğurbil K . B(1) destructive interferences and spatial phase patterns at 7 T with a head transceiver array coil. Magn Reson Med. 2005 Dec;54(6):1503-18.
- Runge JH, van der Kemp WJ, Klomp DW, Luijten PR, Nederveen AJ, Stoker J. 2D AMESING multi-echo (31)P-MRSI of the liver at 7T allows transverse relaxation assessment and T2-weighted averaging for improved SNR. Magn Reson Imaging. 2016 Feb;34(2):219-26.
- Purvis LA, Clarke WT, Valkovič L, Levick C, Pavlides M, Barnes E, Cobbold JF, Robson MD, Rodgers CT. Phosphodiester content measured in human liver by in vivo <sup>31</sup> P MR spectroscopy at 7 tesla. Magn Reson Med. 2017 Feb 28.
- Raaijmakers AJ, Luijten PR, van den Berg CA. Dipole antennas for ultrahigh-field body imaging: a comparison with loop coils. NMR Biomed. 2016 Sep;29(9):1122-30.
- Wijnen JP, Klomp DW, Nabuurs CI, de Graaf RA, van Kalleveen IM, van der Kemp WJ, Luijten PR, Kruit MC, Webb A, Kan HE, Boer VO. Proton observed phosphorus editing (POPE) for in vivo detection of phospholipid metabolites. NMR Biomed. 2016 Sep;29(9):1222-30.
- Valkovič L, Dragonu I, Almujayyaz S, Batzakis A, Young LAJ, PurvisLAB, Clarke WT, Wichmann T, Lanz T, Neubauer S, Robson MD, Klomp DWJ, Rodgers CT. Using a whole-body 31P birdcage transmit coil and 16-element receive array for human cardiac metabolic imaging at 7T. PLoS One. 2017 Oct 26;12(10):e0187153.
Figures
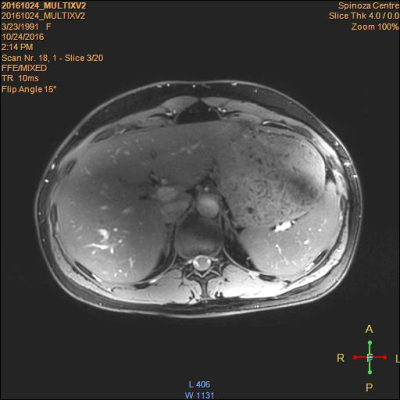
Fig.1 DIXON of
volunteer using 8-antennas and parallel transmit (M2D gradient echo, FOV: 234 x
80 x 359 mm; 256 x 256 x 20 slices; 109 s acquisition, TR/TE1/TE 10/2.6/0.5 ms,
FA=15°, 3 acquisitions).

Fig.2 Penetration
depth of a single antenna shown in a phantom with 20-cm height filled with an
ethylene glycol solution with
1g NaCl per liter.
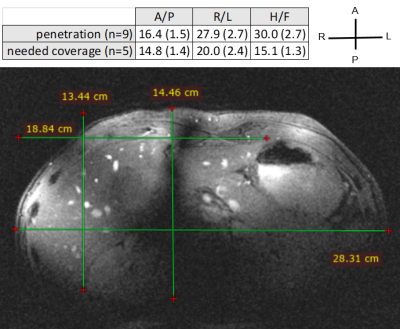
Fig.3 Although insufficient
for radiological grade imaging, it is possible to image the liver for B0-shimming
and placement of the CSI matrix. Gradient echo: 40-degree flip angle, 300 x 330
x 330 mm, 0.8 x 0.9 x 5 mm, TR/TE 4.97/2.42 ms, acquired with 2 antennas (fixed-90-degree
phase). Penetration depths and needed coverage are presented table as mean(std)
across volunteers, and the corresponding measurements are superimposed on the
image: 28.3 cm L/R coverage (18.8 cm length of liver), and 14.5 cm A/P coverage
(13.4 cm depth of liver).
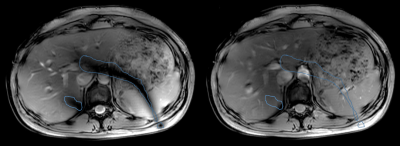
Fig.4 Radiological grade anatomical imaging is
possible with parallel-transmit B1-shimming using 8 antennas. Proton density
MRI before (a) and after (b) B1 shimming. Notice the signal void band (outlined
in blue) due to destructive interference, which is gone on (b) after
calculating and applying the relative transmit-phase offsets for each element.
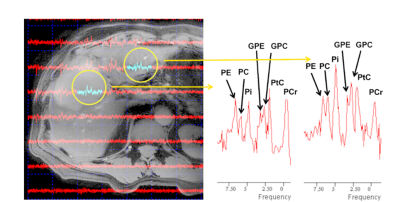
Fig.5
31P 3D CSI (8x8x8 matrix of 30 mm isotropic voxels, TR/TE: 700/0.4 ms)
from a patient with liver metastasis (gastric primary), displayed on a DIXON
image (same protocol as Fig.1) acquired with parallel transmit. Notice the
altered levels of phosphoetanolamine (PE), phosphocholine (PC),
glycerophosphocholine (GPC) and glycerophosphoetanolamine (GPE) in tumor
(left), when compared to normal liver tissue (right), in particular the PE peak
height relative to GPE+GPC.