3834
Monitoring the Growth of Individual Tumours in the Mouse Lung using Cardio-Respiratory Synchronised bSSFP MRI1CRUK and MRC Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, United Kingdom, 2Department of Oncology, University of Oxford, Oxford, United Kingdom
Synopsis
bSSFP imaging is used in-vivo to image small tumours in the mouse lung, at an isotropic resolution of 200µm in under 10 minutes per scan. Cardio-respiratory synchronisation using prospective gating control is used to minimise motion artefacts efficiently, and a 4 step RF phase cycle is used to eliminate banding artefact. Growth of individual tumours in multi-focal lung cancer can be measured with a throughput of >4 mice per hour.
Introduction
The examination of lung tumour growth in the mouse requires fast and high-resolution imaging that is desensitised to cardiac and respiratory motions. Retrospective gating methods have been used 1, 2 but these are necessarily slow due to the repeated acquisition of single k-space data. We have previously shown that prospective gating control can be used to accelerate the production of high-resolution bSSFP images of the mouse lung3, and we now show that the technique is sensitive to the detection of small lung tumours, and their growth.Methods
MRI was performed at 7 T (Varian VNMRS), using a 25 mm diameter RF coil. Cardio-respiratory-gated constant TR bSSFP scanning (TR: 2.8ms; TE: 1.4ms; RF hard pulse: 16µs; FA:30°; FOV: 51.2 x 25.6 x 25.6 mm3; Matrix: 256 x 128 x 128; 32 k-lines per R-wave; 4 phase-cycled images) was performed with phase-cycled bSSFP data combined using the elliptical model.4 Scan time was <10 minutes per mouse and turnaround time was <15 minutes per mouse.
Autochthonous tumour induction was performed in female A/J mice by injecting urethane (10% in PBS, 10 µl/g body weight, i.p., n=6) or equivalent dosing of vehicle (PBS, n=6). Imaging was performed at 8, 13 and 18 weeks post injection. Anaesthesia was induced and maintained using 1-4% isoflurane in oxygen-enriched air, so as to maintain respiration rate at 40-60 breaths/minute. Rectal temperature was maintained at 35-36° C. ECG was detected using subcutaneously implanted needles positioned in the lower abdomen, and respiration was monitored using a pressure balloon. ECG and respiration signals were used to generate gating control signals.
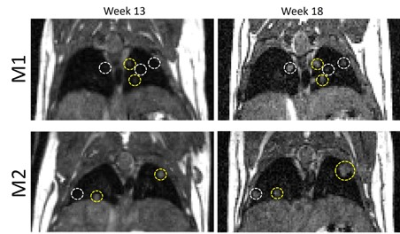
Images from different time points were aligned using threshold-based segmentation masks (ITK-SNAP and MatLab) and tumour volumes were determined via threshold-based segmentation (ITK-SNAP).5
Results and Discussion
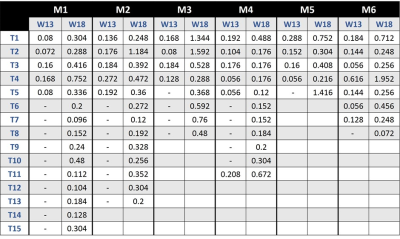
No PBS-treated mice had detectable tumours at any of the time-points examined, and no tumours were unequivocally identified in animals at week 8 post-induction with urethane. However, at week 13 post-induction, all urethane-treated mice had detectable tumours which further increased in size at week 18. In addition, more tumours were detected at week 18 compared with week 13 (Figure 1). The growth rate of individual lung tumours varied between tumours, even within the same mouse lung. Image registration allowed single tumour growth to be efficiently quantified (Table 1), even with tumour volumes estimated to be 0.056 mm3.
bSSFP scan times, for an isotropic resolution of 200 μm, were all below 10 minutes. Including shimming, frequency offset, and RF pulse calibrations the turnaround time per mouse was under 15 minutes, enabling a throughput exceeding 4 mice per hour. Such high throughput provides opportunities that include the use of large sample sizes, more sample groups, more repeated imaging, improved access to limited-availability scanners, and the ability to operate the adaptive trial study design, optimizing experimental and welfare outcomes.
Conclusion
Prospectively gated, cardio-respiratory synchronised bSSFP imaging allows high throughput measurement of individual tumour growth in a mouse model of multi-focal lung cancer.Acknowledgements
This work was supported financially by Cancer Research UK (C5255/A12678, C2522/A10339), and Medical Research Council Unit Grant for Oxford Institute for Radiation Oncology.References
1. Bishop J, Feintuch A, Bock NA, Nieman B, Dazai J, Davidson L, et al. Retrospective gating for mouse cardiac MRI. Magn Reson Med. 2006;55(3):472-7.
2. Tibiletti M, Bianchi A, Kjorstad A, Wundrak S, Stiller D, Rasche V. Respiratory self-gated 3D UTE for lung imaging in small animal MRI. Magn Reson Med. 2017;78(2):739-45.
3. Kinchesh P, Gilchrist S, Gomes A, Kersemans V, Beech J, Allen D, Smart S. Accelerated Imaging of the Mouse Body Using K-Space Segmentation, Cardio-Respiratory Synchronisation and Short, Constant TR: Application to B-SSFP. Proc Intl Soc Mag Reson Med 2016;24:1825.
4. Xiang QS, Hoff MN. Banding artifact removal for bSSFP imaging with an elliptical signal model. Magn Reson Med. 2014;71(3):927-33.
5. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116-28.
Figures