3821
Whole body 3.0 T MRI for Staging Lymphomas: An Assessment of Multiple Sequences Compared to Reference Standard Imaging1Centre for Medical Imaging, University College London, London, United Kingdom, 2Department of Radiology, University College London Hospital, London, United Kingdom, 3Department of Medical Physics and Biomedical Engineering, University College London Hospital, London, United Kingdom, 4Institute of Nucear Medicine, University College London Hospital, London, United Kingdom, 5Department of Haemato-oncology, University College London Hospital, London, United Kingdom
Synopsis
In lymphomas, whole-body MRI (WB-MRI), integrating structural / functional MRI sequences, offers an alternative radiation-free imaging method to standard radiological techniques. In this work, we evaluated multiple MRI sequences as part of a WB-MRI protocol for staging of 22 newly-diagnosed Hodgkin’s lymphoma and diffuse large B-cell lymphoma patients compared to reference-standard 18F-FDG PET-CT. We found that the performance of WB-MRI for nodal / extra-nodal disease detection and Ann-Arbor staging were at best when the entire protocol was reviewed. We observed an inferior diagnostic performance of WB-MRI using diffusion-weighted-imaging and an improved diagnostic performance when T2-weighted / post-contrast WB-MRI were reviewed.
Introduction
Whole body MRI (WB-MRI), integrating structural and functional MRI sequences, offers an alternative radiation-free imaging method to current standard radiological techniques for cancer imaging pathways. In lymphomas, previous works have investigated WB-MRI against current gold standard imaging, 2-deoxy-2-(18F) fluoro-D-glucose positron-emission-tomography fused with computer-tomography (18F-FDG PET-CT) scan [1, 2]. Despite promising initial results [1-3], there are conflicting reports on diagnostic performances of different WB-MRI protocols [4, 5] and, currently, there is no consensus on appropriate WB-MRI protocol. In this work, we evaluated multiple MRI sequences as part of a WB-MRI protocol for staging of newly diagnosed Hodgkin’s lymphoma (HL) and diffuse large B-cell lymphoma (DLBCL).Material and Methods
Twenty-two patients (male/female 12/10, median age 32, range 22-87) with biopsy proven lymphoma (HL/DLBCL 14/8) were prospectively recruited and underwent 3.0 T WB-MRI. Axial T2-weighted turbo-spin-echo (TSE), axial diffusion-weighted-imaging (DWI), dynamic-contrast-enhanced (DCE) MRI of liver/spleen and contrast-enhanced (CE) lung MRI were supplemented by coronal pre- and post-contrast mDixon imaging (Figure 1). For each patient, two radiologists, blinded to other investigations, independently reviewed 4 components of WB-MR datasets:
1. WB-MRI T2 : whole-body T2-TSE
2. WB-MRI DWI+IP : whole-body pre-contrast in-phase (IP) mDixon + whole-body DWI (b1000)
3. WB-MRI Post-C : whole-body post-contrast water-only mDixon, DCE liver/spleen and CE lung
4. WB-MRI All : the entire protocol
Each component was reviewed in a random paradigm with a minimum of 2-weeks wash-out period between readings for the same patient. For each component, the disease status for 18 nodal sites 14 extra-nodal sites as well as final Ann-Arbor stage were derived. For lymph nodes, positivity was defined as short-axis diameter ≥ 10 mm. The criteria used for extra-nodal involvement were as described previously [6]. A 6-point scale was used to score confidence of disease presence in nodal and extra-nodal sites (1: definitely unlikely, 2: highly unlikely, 3: unlikely, 4: likely, 5: highly likely, 6: definitely likely). Scores ≥ 4 were considered positive for disease presence. After the completion of radiologists’ independent reads for entire cohort, a consensus meeting was held between the two reporting radiologists where only discrepant sites of disease were re-evaluated and an agreement was reached. 18F-FDG PET-CT images were reviewed by 2 nuclear medicine physicians in consensus and blinded to other investigations. Disease positivity was defined as the presence of focal FDG uptake greater than that of the surrounding background in a location incompatible with normal physiologic activity, nodal short-axis dimension ≥ 10 mm and maximum-standardized-uptake value ≥ 2.5 using the same 6-point scale described above. A retrospective enhanced-reference-standard (ERS) were derived for nodal/extra-nodal sites by an unblinded expert panel who had access to all available baseline and follow-up imaging/non-imaging data. For each component, the sensitivity, specificity, positive-predictive-value (PPV) and negative-predictive-value (NPV) of WB-MRI for nodal / extra-nodal staging were derived against the ERS. Agreement between the WB-MRI and the ERS for Ann-Arbor stage was tested using kappa statistics.
Results
Across the cohort, 633 disease sites (390 nodal / 243 extra-nodal sites) were evaluated. There were 52 nodal and 9 extra-nodal positive sites according to ERS. The sensitivity, specificity, PPV and NPV for nodal and extra-nodal staging for each reader and consensus reads of all 4 components of WB-MRI protocol is summarised in Figure 2 and Figure 3, respectively. Against the ERS, there were 7 false negative nodal sites due to failure in detection of 18F-FDG avid sub-centimeter lymph nodes by the consensus WB-MRI (Figure 4). The kappa agreement for Ann-Arbor staging for each reader and for consensus read of all 4 component of WB-MRI protocol against the ERS is summarised in Figure 5. The consensus WB-MRI All read had perfect agreement with ERS for Ann-Arbor staging [kappa= 1.00 (95% Confidence interval: 1.00-1.00)].Discussion and Conclusion
In this study, we found that the performance of WB-MRI for nodal and extra-nodal disease detection and Ann-Arbor staging were at best when the entire protocol (WB-MRI All) was reviewed. We also observed an inferior diagnostic performance of WB-MRI DWI+IP imaging for both readers and for the consensus read. Tsuji et al [7] reported that agreement with 18F-FDG PET-CT for staging lymphoma increased from 78% to 93% when T2-weighted MRI was supplemented to DWI only WB-MRI. Similarly, we observed an improved diagnostic performance and agreement for WB-MRI T2 compared to ERS. In conclusion, we demonstrated that a combination of MRI sequences is the best approach for WB-MR imaging with perfect agreement for Ann-Arbor staging in our cohort. However, care should be taken if/when DWI MRI is used as a stand-alone sequence for WB-MR imaging protocol.Acknowledgements
This work was undertaken at the Biomedical Research Centre (BRC), University College Hospital London (UCLH), which received a proportion of the funding from the National Institute for Health Research (NIHR). The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health.
AL was supported by a Cancer Research UK/ Engineering and Physical Sciences Research Council (CRUK/EPSRC) award (C1519/A10331 and C1519/A16463) from the University College London/King’s College London (UCL/KCL) Comprehensive Cancer Imaging Centre (CCIC).
References
[1] Mayerhoefer ME, Karanikas G, Kletter K, et al. Evaluation of Diffusion-Weighted MRI for Pretherapeutic Assessment and Staging of Lymphoma: Results of a Prospective Study in 140 Patients. Clin Cancer Res. 2014;20(1):2984-2993.
[2] Regacini R, Puchnick A, Shigueoka DC, et al. Whole-body diffusion-weighted magnetic resonance imaging versus FDG-PET/CT for initial lymphoma staging: systematic review on diagnostic test accuracy studies. Sao Paulo Med J. 2015;133(2):141-150.
[3] van Ufford HM, Kwee TC, Beek FJ, et al. Newly diagnosed lymphoma: initial results with whole-body T1-weighted, STIR, and diffusion-weighted MRI compared with 18F-FDG PET/CT. Am J Roentgenol. 2011;196(3):662-669.
[4] Kwee TC, Vermoolen MA, Akkerman EA, et al. Whole-body MRI, including diffusion-weighted imaging, for staging lymphoma: Comparison with CT in a prospective multicenter study. J Magn Reson Imaging, 2014;40(1): 26–36.
[5] Siegel MJ, Jokerst CE, Rajderkar D, et al. Diffusion-weighted MRI for Staging and Evaluating Response in Diffuse Large B-cell Lymphoma: A Pilot Study. NMR Biomed.2014;27(6):681-691.
[6] Punwani S, Taylor SA, Bainbridge A, et al. Pediatric and adolescent lymphoma: comparison of whole-body STIR half-Fourier RARE MR imaging with an enhanced PET/CT reference for initial staging. Radiology. 2010;255(1):182-190.
[7] Tsuji K, Kishi S, Tsuchida T, et al. Evaluation of staging and early response to chemotherapy with whole-body diffusion-weighted MRI in malignant lymphoma patients: A comparison with FDG-PET/CT. J Magn Reson Imaging. 2015;41(6):1601-1607.
Figures
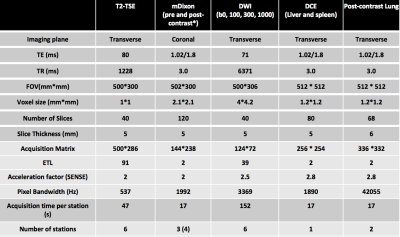
Figure 1: Whole-body MRI sequence parameters
T2-TSE: T2-weighted Turbo Spin Echo, mDixon: modified Dixon, DWI: diffusion Weighted Imaging, DCE: dynamic contrast enhanced, TE: time of echo, TR: time of repetition, FOV: field of view, ETL: echo train Length, SENSE: sensitivity encoding.
* Contrast agent 20 ml intravenous gadoterate meglumine, Dotarem, Guerbet, France
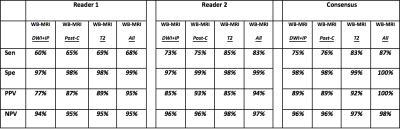
Figure 2: Nodal assessment. Comparison of different MRI sequences as part of the WB-MRI protocol for nodal disease evaluation
DWI+IP: whole body diffusion weighted imaging + pre-contrast in-phase mDixon
Post-C: whole body post-contrast water only mDixon + dynamic contrast enhanced liver and spleen + contrast enhanced lung
T2: whole body T2 weighted turbo spin echo
All: whole body MRI with all available sequences
Sen: sensitivity
Spe: specificity
PPV: positive predictive value
NPV: negative predictive value
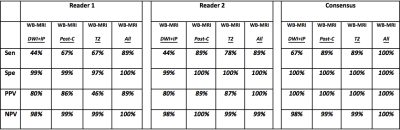
Figure 3: Extra-nodal assessment. Comparison of different MRI sequences as part of the WB-MRI protocol for extra-nodal disease evaluation
DWI+IP: whole body diffusion weighted imaging + pre-contrast in-phase mDixon
Post-C: whole body post-contrast water only mDixon + dynamic contrast enhanced liver and spleen + contrast enhanced lung
T2: whole body T2 weighted turbo spin echo
All: whole body MRI with all available sequences
Sen: sensitivity
Spe: specificity
PPV: positive predictive value
NPV: negative predictive value
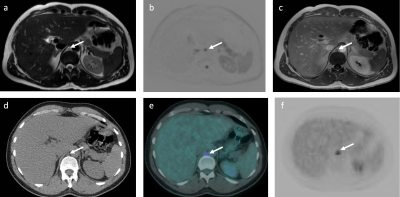
Figure 4: Images of 46-year-old male patient with Hodgkin’s lymphoma highlighting a false negative technical error on WB-MRI.
A sub-centimeter FDG avid (SUVmax 5.1) retrocrural lymph node that was considered negative nodal site on WB-MRI. The positive nodal station is shown (arrows) on (a) T2-TSE, (b) DWI b1000 and (c) CE mDixon in-phase WB-MRI images and (d) CT scan, (e) fused 18F-FDG PET-CT and (f) 18F-FDG PET images.
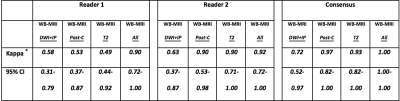
Figure 5: Kappa agreement.
Evaluation of different MRI sequences as part of the WB-MRI protocol for Ann-Arbor staging compared to enhanced reference standard staging
DWI+IP: whole body diffusion weighted imaging + pre-contrast in-phase mDixon
Post-C: whole body post-contrast water only mDixon + dynamic contrast enhanced liver and spleen + contrast enhanced lung
T2: whole body T2 weighted turbo spin echo
All: whole body MRI with all available sequences
95% CI: 95% confidence interval
* weighted kappa