3815
Respiratory-Resolved Single-Shot Fast Spin Echo with Improved Volume Consistency1Global MR Applications & Workflow, GE Healthcare, Menlo Park, CA, United States, 2Global MR Applications & Workflow, GE Healthcare, New York, NY, United States, 3Global MR Applications & Workflow, GE Healthcare, Hino, Japan, 4Global MR Applications & Workflow, GE Healthcare, Houston, TX, United States
Synopsis
We present a motion-correction-based method for improving through-slice volume consistency for respiratory-resolved single-shot fast spin echo for the purposes of image-guided radiation therapy planning.
Introduction
In Image Guided Radiation Therapy Planning (IgRTP), dose planning relies on the accurate estimation of target motion, which needs to be delineated across the whole breathing cycle. In this context, MR can be a valuable tool since it can provide both respiratory-resolved imaging as well as superior soft-tissue contrast1. Previously, a method based on 2D multi-slice multi-phase Single-Shot FSE (SSFSE) was proposed2,3. Through-slice motion consistency remains challenging for 2D approaches4. Indeed, it would require a very long scan time to obtain each slice at each precise respiratory phase of the breathing cycle. In this preliminary study, we propose to resolve the residual motion inconsistencies by modeling and compensating the motion to reach each phase of the respiratory-resolved 4D SSFSE.Methods
Acquisition: Following informed consent, and under IRB approval, a volunteer was scanned under free-breathing conditions on a 60-cm 3.0T MRI scanner (Discovery MR750, GE Healthcare, Waukesha, WI), using a 32-element phased-array, and a previously-presented multi-slice multi-phase single-shot fast spin echo-based pulse sequence with interleaved cylindrical navigator2,3. Respiratory bellows data was also collected, and imaging parameters were as follows: TE = 81 ms, FOV = 40 x 40 cm, 5 mm slice, 1 mm slice overlap (20%), 224 x 192 matrix, ±125 kHz receive bandwidth, 0.71 partial Fourier factor, 2x acceleration (ARC, GE Healthcare, Waukesha, WI), and refocusing flip angle targets of 130, 90, 100 and 45 degrees, respectively. Total imaging time was 10 minutes for 30 cine images at 21 slice locations, for a total of 630 images.
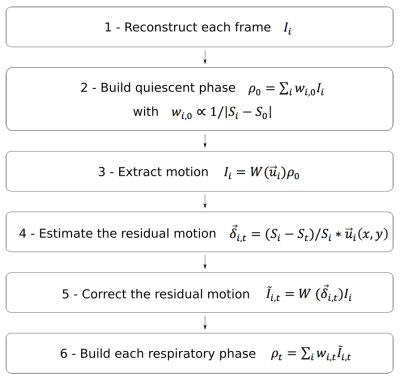
Reconstruction: The ultimate goal of the reconstruction is to adjust each acquired frame to each target phase of the breathing cycle. The reconstruction flows through a 6-step pipeline for each geometrical slice (Figure 1), summarized as follows: (1) Reconstruct each Single-Shot frame individually. (2) Build the quiescent phase (most stable expiration phase) with a weighted average for all the frames. The weights are determined based on the navigator signal. (3) Extract the displacement field between the quiescent phase and each frame (2D non-rigid registration). (4) For each target phase, estimate the residual motion to be applied on each frame to reach the target. Indeed, it can be shown that the motion can be described by a linear model of the physiological signal. (5) Now, each frame can be registered to each target phase. (6) Finally, for each target phase, calculate the weighted average of the motion adjusted frames, the weights being based on the navigator signal distance.
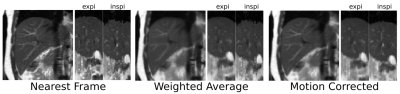
In addition to the above Motion Compensated (MC) 4D reconstruction, two other 4D reconstructions were analyzed, including the Nearest Neighbor frame (NN), and the weighted average (WA) for each bin/slice based on the navigator signal distance.
Analysis: Finally, the three 4D reconstructions were compared using the following criteria: Total Variation (TV) through slice on the diaphragm profile, SNR, and qualitative sharpness.
Results & Discussion
Respiratory-resolved results for the three 4D reconstruction methods are presented in Figure 2. Qualitatively, sharpness appeared to be degraded in the case of WA, but comparable to NN for MC. Analysis of TV through slice demonstrates that MC (0.82) and WA (0.97) were approximately equal, though markedly better than NN (1.24). Likewise, SNR was approximately equal for WA (58.0) and MC (57.4), though significantly higher than in the case of NN (30.7). The quiescent phase appears consistent for all 3 methods, indicating that the scan time was long enough to properly capture this relatively stable phase (Figure 2: NN vs. WA vs. MC: quiescent in-plane coronal + quiescent sagittal reformat + inhale sagittal reformat). The motion inconsistencies through slice displayed in NN and WA are visibly recovered with MC. These preliminary results suggest that the Motion Corrected reconstruction MC recover slice-to-slice consistency while improving SNR and preserving sharpness / mitigating blurring. Consistency is improved because the MC method relies on an absolute / common physiological reference to determine the actual respiratory phase of each frame and then warp it to match each target bin precisely.
Conclusions
We have presented a motion-correction-based method for improving through-slice volume consistency for respiratory-resolved single-shot fast spin echo. Future work will focus on the clinical evaluation of this approach.Acknowledgements
No acknowledgement found.References
- McGee KP, Hu Y, Tryggestad E, Brinkmann D, Witte B, Welker K, Panda A, Haddock M, Bernstein MA. MRI in radiation oncology: Underserved needs. Magn Reson Med. 2015 Jul 14.
- Litwiller DV, Tryggestad E, McGee K, Iwadate Y, Estkowski L, Bayram E. Dynamic, T2-Weighted, Single-Shot Fast Spin Echo with Variable Refocusing Flip Angle and Cylindrical Navigator for Retrospective Respiratory Compensation. Proc of ISMRM, Singapore, 2016, #4257.
- Litwiller DV, Tryggestad E, McGee K, Iwadate Y, Estkowski L, Bayram E. Single-Shot, Navigator-Based Approach to Retrospective 4D MRI: balanced-SSFP vs. Single-Shot Fast Spin Echo. Proc of ISMRM, Singapore, 2016, #1866.
- Tryggestad E, Bayram E, Hu Y, Litwiller D, Rettmann D, Walb M, White D, McGee K. Amplitude-probability sorting for single-pass, retrospective 4D-MRI using 2D bSSFP MRI with interleaved cylindrical navigators. Proc of ISMRM, Honolulu, 2017, #4828.
- McLeish K, Hill D, Atkinson D, Blackall J, Ravazi R. A study of the motion and deformation of the heart due to respiration. IEEE Trans Med Imag 2002; 21:1142-1150